Chapter 87 Prevention of Sudden Cardiac Death with Implantable Cardiac Defibrillators and Cardiac Resynchronization Therapy
Introduction
Sudden cardiac death (SCD) can be defined as cessation of myocardial function within 1 hour of the onset of symptoms. Cardiac arrest leading to SCD is usually assumed to be caused by ventricular tachycardia (VT) or ventricular fibrillation (VF). However, SCD occurs from other causes, including bradycardia, pulmonary embolus, aortic dissection, and other noncardiac conditions. In the United States, SCD is the leading single cause of death and the second leading cause of death after all cancers combined.1 It claims nearly 325,000 lives annually, accounting for one death every 97 seconds. Globally, it accounts for more than 3 million deaths each year. Only 5% of the victims of SCD survive; for every minute in SCD without defibrillation, the mortality rate rises by as much as 10%.2,3 The average arrival time for the typical first responder after collapse is 7 to 8 minutes or even longer.4 Of those who survive to hospital admission, only 3% to 28% are ultimately discharged.5 In 1947, thoracic surgeon Claude Beck was credited as being the first individual to use a cardiac defibrillator successfully when he saved a 14-year-old boy who developed intraoperative VF during thoracic surgery.6 Before the advent of implantable devices, the mainstay of therapy to reduce the incidence of ventricular arrhythmias was primarily with Vaughan Williams class I and III antiarrhythmic drugs (AADs). However, these drugs have consistently failed to show a benefit in reducing SCD. More than 30 years later, in 1980, Mirowski and Mower were instrumental in developing the first implantable cardioverter-defibrillator (ICD) for humans.7 Today, rapid defibrillation is the sine qua non of therapy aimed at restoration of a stable rhythm in those in VT or VF. Additional reductions in the incidence of SCD among high-risk patients are observed with the appropriate use of β-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), aldosterone antagonists, aspirin, and statins. Initially, multiple randomized clinical trials proved that ICDs were superior to standard medical therapy, including AADs, in the secondary prevention of SCD.8–12 For primary prevention of SCD in several high-risk patient groups, ICDs have also been shown to be superior to optimal medical therapy (OMT) (Table 87-1).13–18 In high-risk patients with prolonged intraventricular conduction and progressive heart failure, biventricular pacing with or without ICD backup has been proven to offer additional benefit. Several well-designed clinical trials have shown reduction in SCD and all-cause mortality with cardiac resynchronization therapy (CRT) with or without the addition of cardioverter defibrillator capability.18–21 As with all clinical trials, interpretation of results must be viewed in the context of the patient population studied and the study design in each particular trial. Therefore, this review provides an in-depth summary of the role of ICDs and CRT with or without ICD in the primary and secondary prevention of SCD. Box 87-1 outlines current guidelines for ICD therapy.
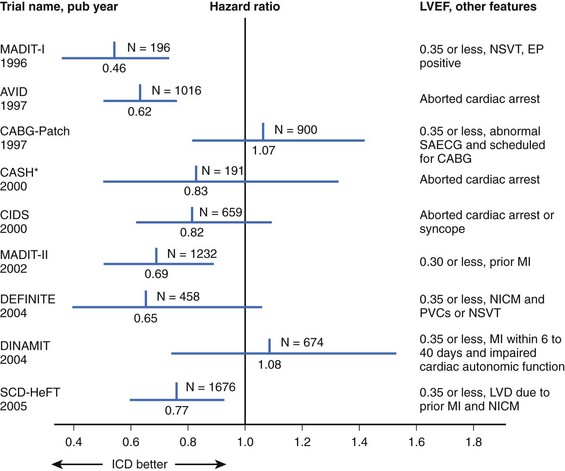
Table 87-1 Major Implantable Cardioverter-Defibrillator Trials for Prevention of Sudden Cardiac Death
Box 87-1 ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy for Prevention of Ventricular Arrhythmias
Class I
Class IIa
Class IIb
Class III
Modified from Epstein AE, DiMarco JP, Ellenbogen KA, et al: ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, J Am Coll Cardiol 51(21):e1–e62, 2008.
Implantable Cardioverter-Defibrillators and Secondary Prevention of Sudden Cardiac Death
Secondary prevention of SCD can be defined as that achieved in those patients who had a previous episode of resuscitated ventricular tachyarrhythmia. The Antiarrhythmics Versus Implantable Defibrillators (AVID) trial, which was published in 1997, was the first of its kind to show that in survivors of VF or sustained ventricular tachycardia causing severe symptoms, an ICD was superior to AADs in increasing overall survival.8 AVID was a multi-center, randomized comparison of single-lead ICD versus amiodarone or, in a few instances, sotolol. The devices used in the trial were single-lead transvenous devices with tiered therapy, defibrillation, cardioversion, bradycardia pacing, and anti-tachycardia pacing (ATP) capabilities. A device without ATP was permitted only if a thoracotomy was otherwise required for device implantation.22 Patients were required to be clinically acceptable for amiodarone therapy to be included in the study. Key inclusion criteria were a history of ventricular arrhythmia as described above and, if VT was the index arrhythmia, an ejection fraction (EF) of 40% or greater. Exclusion criteria were designed to limit the study to patients at high risk for SCD but with life expectancy of greater than 1 year. Key exclusions were New York Heart Association (NYHA) class IV heart failure, inotropic or mechanical support, less than 5 days from revascularization or acute myocardial infarction (AMI), atrial fibrillation (AF), and SCD with a transient or correctable cause. Patients who had transient or reversible causes of SCD and otherwise would have met inclusion criteria were enrolled in a separate AVID registry.
Stratified regression analysis of the results from the AVID registry suggested that the differences in outcomes in the two arms attributable to differences in baseline patient characteristics only accounted for 8% of the relative reduction in the primary endpoint. The imbalance in β-blocker use in the ICD arm was examined by Cox regression analysis, and it was found that this imbalance reduced the beneficial effect of ICD only minimally (unadjusted hazard ratio [HR] for the ICD group of 0.62 compared with adjusted HR of 0.67). The benefit of β-blocker use in congestive heart failure (CHF) has been shown to reduce mortality rate in similar patients in other studies.5 However, an additional benefit may have been observed in patients with paroxysmal AF or sustained VT by reducing inappropriate shocks. Patients in AVID with an index event of VT were more likely to receive shocks or ATP during follow-up. Further retrospective subgroup analysis showed several interesting findings. When the outcomes of those with a left ventricular EF (LVEF) of 35% or less were compared, ICD conferred a 40% relative mortality rate reduction. Of the patients, 39% had an EF less than 35% to 40%. In this subgroup, no statistical benefit in reducing overall mortality rate was observed in the ICD arm.23 The lack of significant mortality benefit may have been caused by a protective effect of amiodarone in this specific population. Previous observational studies have suggested that amiodarone may have a weak protective effect in preventing arrhythmic death and that this benefit may be more significant in patients with higher LVEF.24–26 Spielman et al found that in patients with recurrent sustained VT, higher LVEF and the absence of left ventricular aneurysm or hypokinesis were associated with VT suppressibility.27 In the amiodarone group, survival was strongly correlated with left ventricular systolic function.28 A retrospective analysis of the data from AVID attempted to identify subgroups that had secondary prevention the lowest rates of recurrent ventricular arrhythmias.29 The criteria were VF as the index event, no history of cerebrovascular disease, greater EF, no tachyarrhythmia history, and need for revascularization. Taken together, these findings suggested that important electrophysiological differences exist between these two patient populations.
As previously mentioned, patients screened for AVID but excluded for various reasons were enrolled in a registry. In the AVID registry, after adjusting for multiple variables, patients with transient reversible causes of SCD such as electrolyte abnormalities, new ischemia or infarction, and proarrhythmic drug adverse events actually had poorer outcomes compared with those with primary VT or VF. Patients who were excluded because they had VT that was hemodynamically stable had an overall prognosis similar to that of the patients included in the trial with unstable or pulseless VT.30 These findings argue against the commonly held belief that those with reversible causes of SCD or stable VT have a better prognosis and may not benefit from ICD implantation. Perhaps other clinical characteristics such as the presence of heart failure, stroke, ischemia, or left ventricular dysfunction may be more important discriminators of risk stratification of SCD.
The Canadian Implantable Defibrillator Study (CIDS), which published results in 2000, included a similar study population as AVID. A total of 659 patients were eligible for the study if, in the absence of recent AMI (≤72 hours) or electrolyte abnormalities, they had the following: (1) a history of cardiac arrest, (2) documented sustained VT or VF, (3) sustained VT of at least 150 beats/min, or (4) unmonitored syncope, with depressed LVEF and inducible sustained monomorphic ventricular arrhythmia by programmed ventricular stimulation. Key exclusion criteria were (1) likely survival for less than 1 year, (2) physician discretion against amiodarone or ICD, (3) excessive perioperative ICD implantation risk, (4) previous amiodarone therapy for 6 weeks or more, and (5) long QT syndrome. Patients were randomized to ICD or amiodarone with prespecified stratification by LVEF of 35% or less or greater than 35%. Patients randomized to ICD received a device capable of bradycardia pacing, ATP, cardioversion, and defibrillation. Approxmately 90% of leads were implanted via the transvenous approach, with only 33 undergoing thoracotomy. The primary endpoint was all-cause mortality, and the secondary endpoint was arrhythmic death. Nearly half the patients had VF or cardiac arrest. Similar to AVID, 89% of patients had NYHA class II or less heart failure but, unlike AVID, the population included a small cohort with NYHA class IV heart failure. Ischemic cardiomyopathy was present in 83%. At hospital discharge, β-blockers other than sotolol were prescribed for only 21.4% of patients in the amiodarone arm and 33.5% in the ICD arm, with these differences remaining through follow-up. Less than 10% of the patients were taking class I AADs. However, class I AADs were four times as likely to be used in the ICD arm and have since been shown to increase mortality rate in this patient population.31 Mean duration of follow-up was 2.9 years and 3 years in the amiodarone and ICD cohorts, respectively.
The observed risk reduction in CIDS was less than that of AVID, although both trials were similarly designed. However, substantial overlap exists in the confidence intervals (CIs) of the two studies, suggesting that the results are likely similar. At 1 year, 23.7% of those in CIDS in the AAD arm were taking a β-blocker (including sotolol), which was greater than the 16.8% in AVID. The actual benefit of ICD may have been underestimated in CIDS because of a 20% crossover to ICD in the amiodarone arm as well as the greater use of β-blockers in the ICD arm. As previously noted, data suggest that the combined use of amiodarone and β-blockers may potentiate their antiarrhythmic effects.24,25 The former findings would lessen the impact of ICD benefit in the ICD arm. After the results of CIDS were published, patients were followed up for an average of 5.6 years. The CIDS long-term substudy found progressive increase in benefit of ICD over time.32
The results of CIDS were closely followed by the Cardiac Arrest Study of Hamburg (CASH).10 The study design was more complex than those of previous secondary prevention trials. Enrollment of 407 patients began 4 years before the increased mortality rate associated with class I AADs in patients with structural heart disease was well known.31 Patients were randomized in a 1 : 3 fashion to ICD or medical therapy with one of three drugs intended to reduce ventricular arrhythmias. ICD programming was limited to a shock-only device, with rate as the only criterion for the detection of a sustained ventricular arrhythmia. Within the medical therapy arm, patients were randomized to amiodarone, metoprolol, or propafenone. Inclusion criteria were documented sustained ventricular arrhythmias and aborted cardiac arrest. Exclusion criteria were similar to earlier secondary prevention trials. Patients were excluded if the arrest occurred within 72 hours of AMI, cardiac surgery, electrolyte abnormalities, or proarrhythmic drug effect, or if they had NYHA class IV CHF. More than half the participants had mild heart failure (NYHA class II). In the ICD cohort, 55% of the patients underwent epicardial lead placement via thoracotomy. The index arrhythmia was VF in 84% of patients. The mean LVEF was 46%, with 10% of patients having structurally normal hearts. Another 19% in the ICD arm and 21% in the drug arm underwent revascularization during the index hospitalization for SCD. The propafenone arm was discontinued after 119 patients were enrolled and interim analysis revealed a 61% increase in all-cause mortality in the drug group. This difference was noted at a mean of 11.3 months of follow-up and was clustered in the propafenone arm. The remaining 288 patients enrolled after 1992 were randomized in a 1 : 2 fashion to ICD, amiodarone, or metoprolol. Unlike CIDS and AVID, crossover rates in both groups in this study were relatively low and equal in each arm. In the ICD arm, 5.1% of patients died in the perioperative period versus 1.1% in the drug arm during the same period (P = .029). In addition, the overall complication rate in the ICD arm was 23% through the completed follow-up period. Over a mean follow-up of 57 ± 34 months, the crude death rates were 36.4% in the ICD arm and 44.4% in the drug arm, excluding patients who had been on propafenone. This resulted in a nonsignificant 23% reduction in all-cause mortality (P = .081), with the majority of the benefit in the ICD arm seen in the first 5 years. The ICD group had a significant improvement in survival free of SCD (HR, 0.423, P = .005). The results in the β-blocker arm from CASH helped shed light on previous suggested interaction caused by β-blocker use in the two arms of AVID and CIDS. No significant difference was observed in the crude death rates between the amiodarone and metoprolol groups (29.5% and 35.1%, respectively). As in CIDS, baseline characteristics of patients, lower risk of SCD than predicted, and study design may have limited a demonstrable difference between the ICD and drug arms. Previous studies had suggested those with LVEF greater than 35% may not receive as much benefit from ICD as those with lower LVEF.23 The mean LVEF in CASH was 46% and one tenth of these patients had no organic heart disease. In addition, the observed 2-year overall mortality rate was 19.6%, which was approximately half as high as projected in the original study design. Thus the study was underpowered to detect any significant differences between the two groups. The significant increase in perioperative death in the ICD arm was much higher than current estimates and likely negated any benefit of ICDs on mortality rate.33,34 A nonsignificant trend toward greater benefit with ICD was seen in those with lower LVEF and higher NYHA functional class.
The Midlands Trial of Empiric Amiodarone Versus Electrophysiology-Guided Interventions and Implantable Cardioverter-Defibrillators (MAVERIC) study sought to evaluate the efficacy of electrophysiology study (EPS) in predicting outcomes in patients implanted with ICDs or managed with AADs alone.12 It is important to note that the primary aim of this trial was not to compare ICD versus drug therapy but to determine the role of EPS in this patient population. Patients were randomized to one of two treatment strategies. One group was started on empiric amiodarone therapy. The other underwent a complex EPS-guided algorithm in which implantation of ICD was determined by inducibility of VT or VF as well as the origin of the VT. Those with right ventricular outflow tract VT, bundle branch re-entrant VT, and fascicular tachycardia were referred for ablation and did not undergo ICD implantation. If VT or VF was not inducible by programmed electrical stimulation (PES), a Holter monitor was used to further guide treatment. If the Holter monitor did not reveal more than 30 premature ventricular complexes per hour, an ICD was then implanted. As part of the protocol, all patients were evaluated and treated for ischemia, if present. Survivors of SCD in the absence of AMI in the past 48 hours met the inclusion criteria. Premenopausal women with life expectancy of less than 1 year were excluded. The primary endpoint was all-cause mortality with secondary endpoints of VT or VF recurrence and crossover in treatment. In total, 214 patients were enrolled in the trial. Two important differences existed between this study and AVID, CASH, and CIDS. First, more than half the patients included in this study had hemodynamically stable VT. Second, amiodarone was compared with EPS-guided therapy and not directly with ICD implantation. This study was limited by the small number of patients receiving ICDs (24% in the EPS arm and 5% in the amiodarone arm). More than half the patients had LVEF greater than 35%. The patients in the Massachusetts Veterans Epidemiology Research and Information Center (another MAVERIC) trial were in a high-risk group, and many of those who were not implanted would meet the current class I recommendations for ICD placement. The net outcome of the trial was neutral with no demonstrable difference between empiric amiodarone versus EPS-guided treatment. Despite the limitations of this study, several points were gleaned from this trial. ICD recipients did consistently better than non-ICD recipients with a greater benefit shown by those with hemodynamically unstable ventricular arrhythmias as their index arrhythmia. This contradicts the subgroup analysis from AVID, which showed no difference in ICD benefit based on hemodynamic stability. As in the previous trials, the authors concluded that advanced age, CHF, and LVEF less than 35% were independently associated with death. In addition, diabetes was an independent predictor of poorer outcomes. Nonrandomized comparison of ICD and non-ICD cohorts revealed an HR of 0.54 for percent alive at the end of follow-up. On the basis of study design, these can be only considered hypothesis-generating results. More important, in the setting of secondary prevention, EPS added no additional benefit to conventional treatment strategies. Therefore EPS has no role in determining whether a survivor of sudden cardiac arrest (SCA) will benefit from ICD implantation.
Another secondary prevention trial performed in a more select group of participants was the Defibrillator Versus Beta Blockers for Unexplained Death in Thailand (DEBUT) trial.11 In this two-phase study, survivors of sudden unexplained death syndrome (SUDS) were randomized to ICD or the β-blocker propranolol. As a cohort largely with Brugada syndrome, patients in SUDS tended to have ST elevation in the right precordial leads (V1 to V3) and incomplete right bundle branch block.35 The primary endpoint was all-cause mortality and the secondary endpoints were VT, VF, or cardiac arrest. No differences were observed in baseline characteristics between the two groups, and all patients had structurally normal hearts, with a mean EF of 67%. The results of this trial have somewhat limited applicability because of the significant variability of the underlying pathophysiology of arrhythmia. In this study, 86 patients were randomized to either treatment strategy. The trial was stopped early after 3 years because of an 18% mortality rate in the drug arm and no deaths in the ICD arm. The patients in DEBUT had no contributing factors to death other than VF, which therefore suggests that the ICD fully prevented death.
The results of the aforementioned key secondary prevention trials have been examined further. A meta-analysis published by Connolly et al analyzed the results from AVID, CASH, and CIDS compared with ICD with amiodarone.36 They found significant relative reductions in total mortality rate of 28% and a significant 50% reduction in arrhythmic death. Over a period of 6 years, implantation of an ICD yielded a 4.4-month survival benefit over AAD therapy. The mortality rate reduction with ICD was realized regardless of the presence of structural heart disease, type of presenting arrhythmia (VT or VF), β-blocker use, or prior surgical revascularization. Although benefit occurred in all groups regardless of LVEF, the benefit was greater in those with lower EF. A second similarly designed meta-analysis by Lee et al in 2003 confirmed the findings of Connolly, thus adding to the statistically nonsignificant benefit of ICD implantation in CIDS and CASH.37 A subgroup analysis of the three main secondary prevention trials performed by Oseroff, Retyk, and Bochoeyer found that ICDs confer a 28% RR reduction in all-cause mortality. This benefit was greatest in those with LVEF 20% to 34%. Similar to the findings of MAVERIC, those with inducible VT or VF had no worse mortality rates than those who were noninducible with PES at EPS.38
Primary Prevention of Sudden Cardiac Death
The results of previous secondary prevention trials showed a benefit of ICDs in reducing the incidence of SCD. However, most patients do not survive SCD, so it was logical to explore the use of ICDs for primary prevention in high-risk patients with no history of sustained ventricular arrhythmias (Figure 87-1). The first major published study of primary prevention of SCD was the Multicenter Automatic Defibrillator Implantation Trial (MADIT), published in 1996.13 In this trial, patients with ischemic cardiomyopathy with an EF of 35% or less and NYHA class I to III heart failure, a history of nonsustained VT (NSVT), and inducible nonsupressible sustained VT by PES were randomized to ICD or OMT. The primary endpoint was all-cause mortality. Notable exclusion criteria were surgical revascularization in the past 2 months, angioplasty in the preceding 3 months, recent MI (≤3 weeks), criteria for ICD by secondary prevention standards, and life expectancy of less than 1 year. Of the patients, 253 patients had inducible VT that could not be suppressed by intravenous procainamide, and ultimately 196 were then randomized. Mostly, monophasic pulse generators were used, and roughly half the devices were transthoracic implants (before the advent of transvenous systems). Baseline characteristics of the patients were similar, with a mean EF of 26%. Two thirds of patients in each group had a history of bypass surgery. One month after enrollment, amiodarone was the primary AAD in the OMT arm. The use of ACE inhibitors was similar between groups (60% and 55%). In addition, the overall use of β-blockers was low (25%) but more common in the ICD arm. Sixteen crossovers to ICD occurred. Eleven patients in the OMT arm underwent ICD implantation, and five patients in the ICD arm never had an ICD placed. Two patients had their devices inactivated during the trial. Perioperative mortality rate was 0%, and follow-up was completed in 92% of the ICD group and 86% in the OMT group. Over a mean follow-up of 27 months, an overall mortality rate of 39% occurred in the medical therapy arm and 16% in the ICD arm (HR, 0.46; 95% CI, 0.26 to 0.82; P = .009). This difference translated into a number needed to treat (NNT) of 3 over 36 months to save one life. Sixty percent of those in the defibrillator group received shocks at 2 years, although the appropriateness of these shocks was not well established given the absence of stored electrograms in many devices. This rate is significantly higher than contemporary estimates of appropriate shocks, and it is now known that ICD shocks predict an increase in mortality rate regardless of cause.39 The majority of the differences in death rates were seen in reduction in primary arrhythmia of unknown cause (occurred outside the monitored setting in the OMT arm). It is unclear if the differences in nonarrhythmic deaths were caused by a misclassification of cause of death or the possible adverse effects of AADs. A nonsignificantly greater overall mortality rate at 1 month was observed in the amiodarone group compared with the defibrillator group (36% and 26%, respectively). However, amiodarone was the most used AAD, and other data suggest that it can be used without excess mortality from heart failure.40 A Cox regression analysis failed to attribute the difference in the two groups to differences in medical therapy. In addition, the observed crossover would have only diluted observed differences in outcome between the two groups.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree