Prevention of Neurologic Injury after Coronary Artery Bypass
George J. Arnaoutakis
William A. Baumgartner
INTRODUCTION
Coronary artery bypass grafting (CABG) increases long-term survival compared with medical therapy for patients with significant coronary artery disease. Improvements in anesthesia, surgical technique, and myocardial protection have led to improved operative mortality rates. Despite these improvements, neurologic injury remains a significant risk for patients undergoing CABG. Aside from being a leading cause of morbidity in CABG patients, neurologic sequelae also account for escalating medical costs in the form of increased length of hospital stay and subsequent rehabilitation.
The spectrum of neurologic injury ranges from clinical stroke to more prevalent, yet subtle neurocognitive changes. According to a recent Society of Thoracic Surgeons (STS) report clinically evident stroke occurs in 1.4% of patients undergoing CABG and is associated with significantly worse early and long-term survival after CABG. Delirium occurs in up to 10% of patients >65 years of age and is also associated with worse long-term mortality. Other early postoperative neurocognitive abnormalities occur in up to 60% of patients and manifest as mild deficits in memory, attention, concentration, and language. Many of these subtle neurocognitive changes are transient, however, and are known to resolve at 3 months post-CABG. The three main etiologic factors involved in neurologic injury are atherosclerotic emboli, cerebral hypoperfusion, and generalized perioperative inflammation. Identifying the mechanism of injury and potentially modifiable risk factors for unfavorable neurologic outcomes is an active area of research, and many potential neuroprotective strategies have emerged.
Preoperative comorbidities predisposing to neurologic complications after cardiac surgery include advanced age, prior neurologic events, hypertension, diabetes, renal failure, atrial fibrillation, peripheral vascular disease, and carotid stenosis. These risk factors identify individuals likely to have diffuse cerebrovascular disease, impaired cerebral blood flow (CBF), or increased susceptibility to thromboembolic events. To screen for patients with asymptomatic carotid stenosis, carotid duplex ultrasonography should be performed in patients with audible bruits or in elderly patients at high risk for cerebrovascular disease.
Studies have also identified intraoperative predictors for perioperative neurologic events. These independent risk factors include the presence of significant aortic arch atherosclerosis, longer duration of cardiopulmonary bypass (CPB), and CABG with concomitant carotid endarterectomy. The preoperative recognition and assessment of risk factors is an important step in reducing the morbidity and mortality associated with perioperative neurologic injury.
MECHANISM OF NEUROLOGIC INJURY
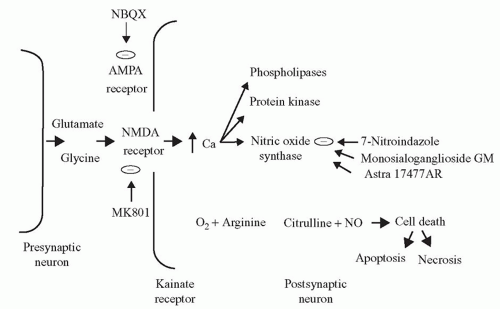
The development of potential therapeutic strategies depends on our understanding of the mechanism of neuronal cell injury following cardiac surgery. “Glutamate excitotoxicity” is a major mechanism of neuronal injury. Glutamate is the major excitatory amino acid neurotransmitter in the central nervous system (CNS) and can cause neuronal hyperactivity and death during periods of metabolic stress such as hypoxia or ischemia (Fig. 39.1). Glutamate triggers a cascade of events via binding to an N-methyl-D-aspartate (NMDA) receptor, ultimately resulting in neuronal necrosis or apoptosis. Histologically, the brain regions most significantly affected are those in which NMDA receptors are prominent: the hippocampus, cerebellum, and basal ganglia. Pharmacologic blockade of this receptor also confers some degree of neurologic protection in animal models, thus reinforcing this mechanism of injury.
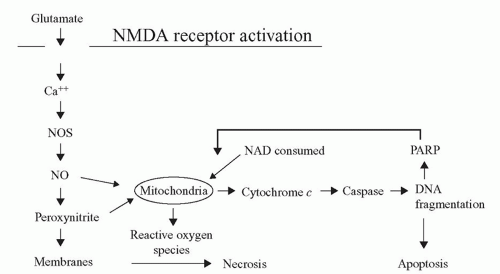
Nitric oxide (NO) is a ubiquitous molecule that also acts as a neurotoxin. Induction of neuronal nitric oxide synthase (nNOS) via a hypoxic/ischemic insult leads to diffuse NO formation in the brain. NO and its metabolite peroxynitrite impart toxic effects on the neuronal mitochondria, resulting in free radical proliferation and DNA fragmentation (Fig. 39.2). Mitochondrial energy failure is considered to play a central role in neuronal cell death. Investigations using a canine model of hypothermic circulatory arrest (HCA) showed that inhibition of nNOS results in decreased NO production and superior neurologic function compared with untreated HCA canines. These studies demonstrated that pharmacologic intervention at specific points in the injury cascade might potentially mitigate the neurologic deficits that can result from cardiac surgery.
CEREBRAL PROTECTION TECHNIQUES
Minimizing the Systemic Inflammatory State
CPB is associated with an intense inflammatory response due to contact of blood with the artificial bypass surfaces, conversion to laminar, nonpulsatile flow, and leukocyte and endothelial cell activation following ischemia/reperfusion. The systemic inflammatory response is characterized by activation of the complement, fibrinolytic, and cytokine cascades. Complement activation occurs immediately after the blood comes into contact with the foreign surfaces of the bypass circuit. This leads to leukocyte activation and increased inflammatory cytokine production, such as interleukin-6 and tumor necrosis factor. The leukocyte-endothelial cell interactions result in microvascular occlusion and end-organ ischemia. This inflammatory response is the suspected mechanism underlying the global cerebral edema seen on post-CPB magnetic resonance imaging (MRI).
Many therapeutic strategies have been used in an attempt to mitigate the CPB-induced inflammation. These include both pharmacologic and mechanical therapeutic modalities. Corticosteroids have been shown to reduce CPB-induced inflammation by decreasing complement activation and levels of circulating cytokines. However, a large Cochrane Database review of over 3,600 patients revealed that the routine use of preoperative corticosteroids in adults did not confer any clinical benefit, including no improvement in neurologic complications. The serine protease inhibitor aprotinin was shown to reduce the inflammatory response post-CPB and possibly decrease postoperative stroke risk. However, due to increased risk of fatal myocardial infarction and renal failure, this drug is no longer available. Experimental evidence in animal models suggests decreased post-CBP inflammatory response in subjects pretreated with statin therapy; however, a large cohort study failed to demonstrate a clinical benefit in neurologic outcomes.
Mechanical strategies to reduce the release of proinflammatory cytokines include leukocyte and hemoconcentration filters, which wash the cells before returning them to the bypass circuit. However, despite several studies documenting reductions in measured cytokine concentrations, significant clinical benefit has not been shown. In an attempt to increase the biocompatibility of CPB, heparin-coated circuits also reduce complement activation, proinflammatory cytokine levels, and neutrophil adhesion. A prospective, randomized trial of heparincoated circuits demonstrated a significant improvement in postoperative neuropsychometric tests as compared with conventional circuits.
Maintenance of Cerebral Perfusion
Under normal physiologic conditions, cerebral autoregulation maintains a constant CBF over a wide range of systemic arterial pressures (50 to 120 mmHg). Below 50 mmHg, cerebral oxygen delivery (CDO2) becomes pressure-dependent. This can be compensated for by an increase in cerebral oxygen extraction. Therefore, CDO2 remains relatively constant even at systemic pressures as low as 30 mmHg during moderate hypothermia. However, because of the burden of comorbidities prevalent in CABG patients, including hypertension, diabetes, and cerebrovascular disease, the ischemic tolerance and autoregulatory capacity of the brain are diminished. For this reason, higher minimal perfusion pressures may be required to support adequate cerebral oxygenation. Thus, flow rates during CPB are adjusted to preserve end-organ and cerebral perfusion above an ischemic threshold.
However, the watershed areas of the brain still remain at greatest risk for neurologic injury. A prospective, randomized trial of 248 elective CABG patients showed that those maintained at lower perfusion pressures (50 to 60 mmHg) had significantly higher mortality and stroke rates than those maintained at a higher perfusion pressure (80 to 100 mmHg). This study demonstrated that maintaining patients on CPB at a higher perfusion pressure was technically safe and effectively improved outcomes after cardiac surgery. The current perfusion practices, which err on the side of higher perfusion pressures, result in excellent outcomes in the vast majority of patients, and there is little evidence to suggest that altering these practices will have a positive influence on CBF.
Embolization Reduction
There are three main types of embolic phenomena that may occur during cardiac surgery and result in postoperative neurologic dysfunction: particulate, gaseous, and lipid embolization. Perhaps, the most significant of these is the particulate emboli resulting from atheromatous debris present in the diseased aorta and great vessels. The platelet-fibrin aggregates and debris generated by the CPB circuit itself also have a causative role. To limit particulate embolization, surgical manipulation of the heart and great vessels must be minimized and care should be taken in the placement of cannulae and clamps on atherosclerotic vessels. In addition to intraoperative palpation, transesophageal echocardiography (TEE) and epiaortic ultrasonography are commonly used to assess the degree of aortic atherosclerosis and to identify more favorable areas for cannulation and clamping. In a recent study, patients undergoing epiaortic scanning showed a lower incidence of cognitive dysfunction than patients evaluated only with aortic palpation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree