Postmortem computed tomography (PMCT) has been recently reported to be useful for detecting causes of death in the emergency department. In this study, the incidence and causes of death of type A acute aortic dissection (AAD) were investigated in patients who experienced out-of-hospital cardiopulmonary arrest (OHCPA) using PMCT. PMCT or enhanced computed tomography was performed in 311 of 528 consecutive patients experiencing OHCPA. A total of 23 (7%) of 311 patients were diagnosed with type A AAD based on clinical courses and CT findings. Eighteen consecutive patients who did not experience OHCPA were diagnosed with type A AAD during the same period. Pre-hospital death was observed in 21 (51%) of 41 patients with type A AAD. Bloody pericardial effusion was observed more frequently in patients who experienced OHCPA with type A AAD than in those who did not experience OHCPA with type A AAD (91% vs 28%, respectively; p <0.05). In conclusion, the incidence of type A AAD was common (7%) in patients who experienced OHCPA, with a high rate of pre-hospital death. Aortic rupture to the intrapericardial space was considered the major cause of death in patients who experienced OHCPA with type A AAD.
Stanford type A acute aortic dissection (AAD) often causes pre-hospital sudden death (61%) and resembles several other disorders, such as myocardial infarction and stroke, which makes it difficult to report the exact incidence of type A AAD. Additionally, autopsy imaging methods, such as postmortem computed tomography (PMCT), have become increasingly popular in recent years because of their accessibility, noninvasiveness, and potential for detecting the cause of death. Therefore, the aim of this study was to investigate the incidence of type A AAD in patients who experienced out-of-hospital cardiopulmonary arrest (OHCPA) and to evaluate the factors associated with OHCPA in emergency settings using PMCT.
Methods
We retrospectively reviewed the database of our emergency department (ED) and searched for patients transferred to the ED because of OHCPA at the Teine Keijinkai Medical Center, 1 of 5 tertiary medical centers in Sapporo, from April 2009 to March 2012. Patients who died of exogenous death, such as trauma or suicide, and those without clinical records were excluded from this study. As part of our standard practices, PMCT was always performed to identify the cause of death when the cause of death could not be identified in the ED. The local research ethics committee approved this study. As stipulated by the research ethics committee, we obtained verbal informed consent from patients’ families for PMCT. The cost of PMCT was incurred by the hospital. No extramural funding was used to support this work.
We recorded the initial data regarding cardiac arrest and OHCPA, such as initial electrocardiographic rhythm, including ventricular tachycardia (VT), ventricular fibrillation (VF), pulseless electrical activity (PEA), and asystole, and demographic data, such as age and gender. Information on the estimated time course between the last witness and discovery was obtained from the appropriate subjects. OHCPA was defined as a state without consciousness, breath, or pulse on arrival of emergency service. Pre-hospital death was defined as a state in which spontaneous circulation was never observed from discovery until the declaration of death in the ED.
In the ED, we performed cardiopulmonary resuscitation (CPR) according to the CPR guidelines of the American Heart Association (2005 and 2010). In parallel with the initial treatment mentioned earlier, physical examinations, blood tests (complete blood counts, biochemistry, and blood gas), electrocardiograms (ECGs), chest radiographs, and echocardiography were also performed. ECGs were evaluated to determine VT/VF, PEA, asystole, and ST-T changes.
PMCT was performed to identify the cause of death when patients died or were expected to not to recover from OHCPA. CT images were acquired on a 16-slice multidetector scanner (Somatom Emotion 16; Siemens AG., Wittelsbacherplatz, Munich, Germany). Contiguous 6-mm axial images were obtained through the brain at 120 kV with 310 mAs, and 7-mm axial images were obtained from the vertex to the symphysis pubis at 120 kV with 160 mAs. We evaluated the amount of pericardial effusion (ml) using volume-rendered CT imaging (AW VolumeShare 2; GE Healthcare UK Ltd., Little Chalfont, Buckinghamshire, UK).
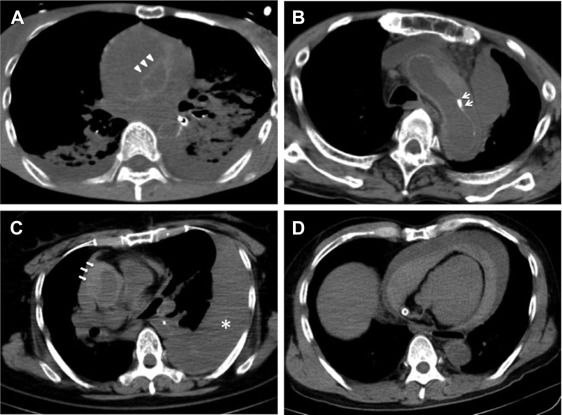
Diagnostic criteria of type A AAD and type B AAD by PMCT were defined based on previous reports. A visible intimal flap ( Figure 1 , arrow), inward shift of the calcified intima ( Figure 1 , arrow), and intramural hematoma ( Figure 1 , arrow) in the ascending aorta (type A) and descending aorta (type B) were adopted as diagnostic criteria because these factors have been reported as useful diagnostic findings in patients with AAD. Diagnosis of type A and type B AAD was made if the patients met both the criterion of acute onset (<2 weeks) and at least one of the PMCT criteria. Evaluation of pericardial effusion ( Figure 1 , arrow) and aortic rupture ( Figure 1 , asterisk) and measurement of the diameter of the ascending aorta were performed after diagnosis of type A AAD. Bloody pericardial effusion was defined as fluid collection in the pericardial space with 30 to 60 Hounsfield units.
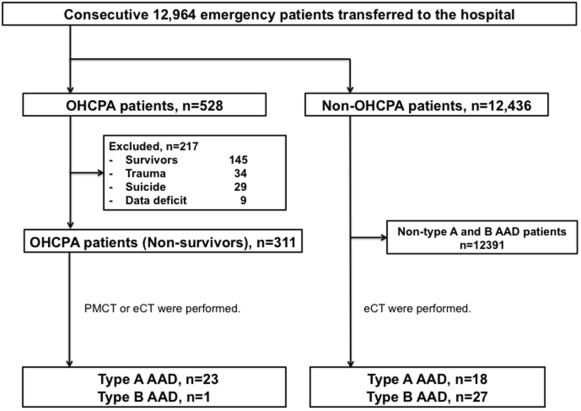
We initially evaluated the characteristics of CT findings in patients who experienced OHCPA with type A AAD and the incidence of pre-hospital death in type A and type B AAD. We separately enrolled both 18 consecutive patients who did not experience OHCPA diagnosed with type A AAD and 27 consecutive patients who did not experience OHCPA with type B AAD who were transferred to the ED during the same period ( Figure 2 ). Then, we investigated factors associated with OHCPA in type A AAD.
Statistical analysis was performed using JMP 11 (SAS Institute Inc., Cary, North Carolina) and EZR statistical software. Categorical data are presented as the frequency and percentage, whereas continuous variables are shown as the mean ± SD or median and interquartile range. Categorical variables were compared using chi-square tests or Fisher’s exact tests. Continuous variables were compared using Student’s t tests or Mann-Whitney U tests. Univariate and adjusted logistic regression analyses by age and gender were performed to reveal associated factors for OHCPA. Differences or associations with p values <0.05 were considered significant.
Results
A total of 12,964 patients were transferred to our hospital from April 2009 to March 2012, and 528 patients who experienced OHCPA were included in this analysis. Of these 528 patients, 217 were excluded because of the following reasons: 145 patients survived, 34 were trauma cases, 29 were suicide cases, and 9 had incomplete records. Among the 145 survivors, 2 patients were hospitalized because of type A AAD diagnosed by enhanced CT, and 1 patient was transferred to another hospital for operation for type A AAD diagnosed by enhanced CT. Among the remaining 311 patients with OHCPA, 5 patients underwent enhanced CT before they died in the ED, and 2 of the 5 patients were diagnosed with type A AAD. Twenty-nine patients did not undergo PMCT. Among these 29 patients, permission to perform PMCT was not obtained in 16 patients. Thus, 277 of 311 nonsurvivors (89%) were subjected to PMCT for evaluation of the cause of death. Twenty-one nonsurvivors were diagnosed with type A AAD by PMCT. As a result, 23 (7%) of 311 patients and 1 (0.4%) of 311 patients were diagnosed with type A and type B AAD, respectively ( Figure 2 ). Eighteen patients with type A AAD and 27 patients with type B AAD did not experience OHCPA. Pre-hospital death was observed in 21 (51%) of 41 patients with type A AAD and 1 (4%) of 28 patients with type B AAD (p <0.01).
PMCT was performed before the declaration of death in 4 patients and a median of 18 min (6 to 33) after the confirmation of death in the remaining 19 patients. The PMCT or enhanced CT findings for 23 nonsurvivors with type A AAD are summarized in Table 1 . Interestingly, bloody pericardial effusion was observed in 21 of 23 patients (91%), although echocardiography showed that 12 (53%) of 23 patients exhibited pericardial effusion. Volume-rendered CT imaging revealed that the median amount of bloody pericardial effusion was 425 ml (interquartile range 246 to 678). Furthermore, we observed 5 patients with bloody pericardial effusion of the 311 patients who experienced OHCPA and did not have type A AAD. The positive predictive value of blood pericardial effusion for type A AAD was 81%.
| Intimal flap or inward shift of intimal calcification in ascending aorta | 8 (35%) |
| Intramural hematoma in ascending aorta | 15 (65%) |
| Bloody pericardial effusion | 21 (91%) |
| Aortic rupture to the thoracic cavity or mediastinum | 6 (26%) |
ECGs performed in the ED showed that asystole and PEA were more frequent than VT/VF (96% vs 4%, respectively). ST changes were observed in 3 of 5 patients with PEA. Fourteen (61%) of 23 patients collapsed in front of someone, and the remaining patients 6 (39%) were found lying down or unconscious within 16 hours from the last witness. Moreover, 17 (74%) of 23 patients were estimated to have collapsed within 10 minutes before discovery; these findings were compatible with acute onset. Nevertheless, only 1 patient exhibited VT/VF.
The basic characteristics of the 23 patients who experienced OHCPA and 18 patients who did not experience OHCPA with type A AAD are summarized in Table 2 . Increased age, cerebrovascular disease, and the presence of bloody pericardial effusion were significant in patients with OHCPA compared with those without OHCPA. Of the 41 patients with type A AAD, 25 died in the ED, and 4 of these 25 patients had no pericardial effusion. Among these 4 patients, 3 showed remarkable ST changes (VF, ST elevation, and ST depression), and the remaining 1 patient had significant cerebrovascular occlusion, as revealed by enhanced CT. The results of logistic regression analysis of factors associated with OHCPA are listed in Table 3 . Adjusted analysis by age and gender revealed that age (>75 years), male gender, and bloody pericardial effusion were independent factors associated with OHCPA.
| Variable | OHCPA (n=23) | Non-OHCPA (n=18) | P |
|---|---|---|---|
| Age (years) | 77 ± 13 | 67 ± 17 | 0.03 |
| Men | 16 (70%) | 6 (33%) | 0.03 |
| Hemoglobin (g/dl) | 13 ± 3 | 12 ± 3 | 0.47 |
| Creatine phosphokinase (IU/l) | 89 (51 – 144) | 82 (41 – 161) | 0.80 |
| Creatinine (mg/dl) | 1.1 ± 0.8 | 1.0 ± 0.8 | 0.73 |
| Hypertension | 10/14 (71%) | 11/17 (65%) | 0.50 |
| Cerebrovascular disease | 5/8 (63%) | 2/15 (13%) | 0.03 |
| Coronary heart disease | 1/8 (13%) | 0/14 (0%) | 0.36 |
| Diabetes mellitus | 3/9 (33%) | 0/11 (0%) | 0.07 |
| Diameter of the ascending aorta (cm) | 44 ± 11 | 49 ± 8 | 0.10 |
| Intramural hematoma of the ascending aorta | 15 (65%) | 6 (33%) | 0.06 |
| Bloody pericardial effusion | 21 (91%) | 5 (28%) | <0.01 |
| Aortic rupture to the thoracic cavity or mediastinum | 6 (26%) | 0 (0%) | 0.03 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


