Surrogate endpoints facilitate trial efficiency but are variably linked to clinical outcomes, and limited data are available exploring their utilization in cardiovascular clinical trials over time. We abstracted data regarding primary clinical, intermediate, and surrogate endpoints from all phase II to IV cardiovascular clinical trials from 2001 to 2012 published in the 8 highest Web of Science impact factor journals. Two investigators independently classified the type of primary endpoint. Of the 1,224 trials evaluated, 677 (55.3%) primary endpoints were clinical, 165 (13.5%) intermediate, and 382 (31.2%) surrogate. The relative proportions of these endpoints remained constant over time (p = 0.98). Trials using surrogate endpoints were smaller (187 vs 1,028 patients) and enrolled patients more expeditiously (1.4 vs 0.9 patients per site per month) compared with trials using clinical endpoints (p <0.001 for both comparisons). Surrogate endpoint trials were independently more likely to meet their primary endpoint compared to trials with clinical endpoints (adjusted odds ratio 1.56, 95% CI 1.05 to 2.34; p = 0.03). Rates of positive results in clinical endpoint trials have decreased over time from 66.1% in 2001 to 2003 to 47.2% in 2010 to 2012 (p = 0.001), whereas these rates have remained stable over the same period for surrogate (72.0% to 69.3%, p = 0.27) and intermediate endpoints (74.4% to 71.4%, p = 0.98). In conclusion, approximately a third of contemporary cardiovascular trials use surrogate endpoints. These trials are completed more expeditiously and are more likely to meet their primary outcomes. The overall scientific contribution of these surrogate endpoint trials requires further attention given their variable association with definitive outcomes.
Randomized clinical trials are the most impactful studies in directing evidence-based clinical care. Given the potential implications of clinical trial results, study design with regard to endpoint selection is an important consideration. Surrogate endpoints (for instance, laboratory or radiographic measurements), which serve as a proxy for more clinically oriented outcomes, facilitate trial efficiency. A number of recent, prominent cardiovascular trial programs have demonstrated early efficacy of the study intervention on surrogate endpoints, which have not been validated on subsequent evaluation of the same intervention in trials assessing clinical outcomes. As such, the overall utility and role of surrogate endpoints in cardiovascular trials have not been entirely defined. Indeed, there is a potential danger in using surrogate endpoints despite the appeal of bringing therapeutics to market earlier. However, at present, limited data exist regarding the utilization of surrogate endpoints over time specifically for cardiovascular disease clinical trials. We sought to examine trends in endpoint selection in contemporary cardiovascular clinical trials published in the 8 highest impact factor major journals from 2001 to 2012.
Methods
We conducted an electronic search of the PubMed database using the following key words: “trial*” and “random*”. This search was restricted to cardiovascular phase II to IV randomized controlled clinical trials that were published from January 2001 to December 2012 in the 8 highest Web of Science impact factor journals in the categories “General Internal Medicine” and “Cardiology,” according to the 2013 edition of the Journal Citation Reports. These journals included Annals of Internal Medicine (Ann Intern Med), British Medical Journal (BMJ), Circulation , European Heart Journal (EHJ), Journal of the American College of Cardiology (JACC), Journal of the American Medical Association (JAMA), Lancet , and New England Journal of Medicine (N Engl J Med). A subsequent manual search of each individual journal edition from January 2001 to December 2012 was also performed to ensure inclusion of all relevant trials during this time period.
Studies evaluated the following cardiovascular topic areas: acute coronary syndrome, adverse medication effects, anticoagulation, arrhythmias, chronic coronary artery disease, heart failure, heart transplantation, lipids, metabolic syndrome, primary and secondary prevention, perioperative cardiovascular care, pulmonary hypertension, peripheral vascular disease, stroke, systemic hypertension, venous thromboembolism, and valvular heart disease. Trials were excluded if they represented (1) interim, secondary, or post hoc analyses; (2) pilot or phase I trials; (3) pediatric trials; or (4) trials with hospitals as units of randomization.
The following data elements were abstracted from each cardiovascular trial identified: (1) journal; (2) year of publication; (3) trial design; (4) type of primary endpoint; (5) intervention; (6) duration of trial; (7) total number of patients enrolled; (8) total number of sites; (9) number of participating countries; (10) funding sources; and (11) whether the trial met its intended primary endpoint. Trial design was categorized as: (1) trials evaluating superiority of 2 therapeutic strategies (superiority); (2) trials designed to address noninferiority or equivalence between 2 therapeutic strategies (noninferiority); and (3) trials evaluating new therapies compared with placebo (placebo-controlled).
The primary variable of interest for the present analysis was primary endpoint classification, which was grouped into 3 categories. Endpoints were categorized based on classification systems that have been reported previously. Representative endpoints are summarized in Table 1 . Clinical endpoints were defined as the onset of disease state (e.g., death, stroke, and myocardial infarction) or hospitalization. Intermediate endpoints were defined as patient symptoms, functional assessment, or change in vital signs (e.g., symptom scores, exercise tolerance, 6-minute walk test, cardiopulmonary exercise testing). Surrogate endpoints were defined as laboratory or radiographic results (e.g., coronary artery calcium scores, infarct size, cardiac biomarkers, and lipid levels). Only primary endpoints were included for categorization. In the case of composite or co-primary endpoints, the presence of a more clinical endpoint took precedence (e.g., if both mortality and 6-minute walk tests were co-primary endpoints, the trial was classified as using a clinical endpoint). Surrogate endpoints included only single or composite surrogate endpoints. Intermediate endpoints included composite endpoints with at least 1 intermediate endpoint (but not clinical endpoint). Clinical endpoints included composite endpoints with at least 1 clinical endpoint. Two investigators (RBP and MV) independently adjudicated category of primary endpoint. If there was discrepancy between reviewers, a third investigator (AS-T) was asked to assist in decision-making. A trial was defined as “positive” by the ability to reject the null hypothesis and support the alternative hypothesis for the primary endpoint (i.e., that intervention was either superior or noninferior/equivalent according to the primary hypothesis reported in the Methods section of the trial publication).
| Clinical Endpoints | Mortality |
| Hospitalization | |
| Myocardial infarction | |
| Worsening heart failure | |
| Stroke/transient ischemic attack/intracranial hemorrhage | |
| Freedom from arrhythmia | |
| Venous thromboembolism | |
| Symptomatic peripheral arterial disease or amputation | |
| Dialysis dependence | |
| Intermediate Endpoints | Quality of life |
| Exercise tolerance test results | |
| Dyspnea | |
| Blood pressure | |
| Heart rate | |
| Peak oxygen consumption | |
| Body weight | |
| Functional status | |
| Treatment compliance | |
| Change in clinical risk scores | |
| Surrogate Endpoints | Biomarker level |
| Laboratory markers | |
| Vessel lumen size | |
| Carotid intimal media thickness | |
| Platelet reactivity | |
| Left ventricular ejection fraction | |
| Left ventricular systolic volume |
Temporal trends were compared across four 3-year periods on the basis of publication date (2001 to 2003, 2004 to 2006, 2007 to 2009, and 2010 to 2012) using nonparametric tests for trend. Enrollment rate was calculated for each trial and expressed as number of patients enrolled per site per month. To identify differences in quantitative characteristics across nominal categories, we used chi-square tests, Kruskal–Wallis tests, and 1-way analysis of variance tests with Bonferroni correction for multiple comparisons, as appropriate. To identify predictors of positive trials, we used multiple logistic regression to estimate odds ratios (ORs) and associated 95% CIs, accounting for major trial characteristics (study design, funding source, year of publication, size of trial, region of enrollment, enrollment rate, cardiovascular category of study, and composite vs single endpoint status). Analyses were performed using STATA, version 14.0 (StataCorp, College Station, Texas).
Results
The electronic and manual journal search used in this study identified a total of 4,524 trials published during the study period. Among the final analytic cohort of 1,224 trials after relevant exclusions ( Figure 1 ), primary endpoints were identified as clinical in the majority (n = 677, 55.3%) followed by surrogate (n = 382, 31.2%) and finally intermediate (n = 165, 13.5%). The relative proportions of endpoint selection remained constant over time from 2001 to 2003 to 2010 to 2012 (p = 0.98 for temporal trend; Figure 2 ).
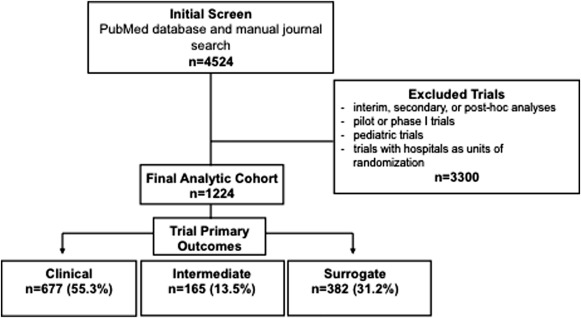
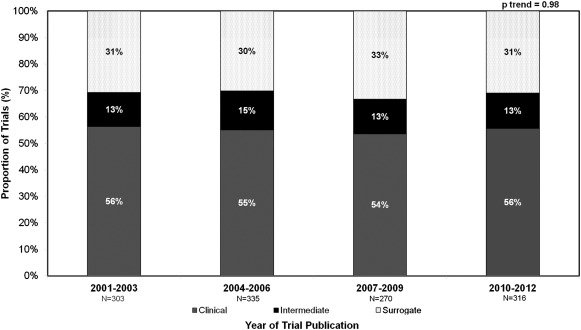
Table 2 displays trial characteristics stratified by primary endpoint. Compared to trials with clinical endpoints, those with surrogate endpoints were smaller (median 187 vs 1,028 patients per trial, p <0.001), were completed over a shorter duration (median 1.6 vs 2.4 years, p <0.001), enrolled patients more expeditiously (median 1.4 vs 0.9 patients enrolled per site per month, p <0.001), and were less likely to have multinational enrollment. There was no difference in the funding sources between trials based on primary endpoints.
| Clinical | Intermediate | Surrogate | p | |
|---|---|---|---|---|
| Number of trials | 677 | 165 | 382 | <0.001 |
| Number of patients | 2,071,236 | 124,656 | 154,740 | <0.001 |
| Trial Size | ||||
| Patient per trial | 1028 (351-3057) | 281 (130-609) | 187 (75-394) | <0.001 |
| Sites per trial | 41 (7-146) | 11 (1-49) | 5 (1-24) | <0.001 |
| Countries per trial | 2 (1-12) | 1 (1-3) | 1 (1-2) | <0.001 |
| Duration (years) | 2.4 (1.5-3.8) | 2.0 (1.1-3.0) | 1.6 (1.0-2.5) | <0.001 |
| Enrollment rate (patients/site/month) | 0.9 (0.4-2.7) | 1.4 (0.4-4.7) | 1.4 (0.7-4.2) | <0.001 |
| Trial Design | <0.001 | |||
| Superiority | 391 (56.7%) | 99 (14.3%) | 200 (29%) | |
| Non-inferiority | 49 (81.7%) | 1 (1.7%) | 10 (16.6%) | |
| Placebo-controlled | 236 (49.9%) | 65 (13.7%) | 172 (36.4%) | |
| Met primary endpoint | 385 (50.5%) | 122 (16%) | 255 (33.5%) | <0.001 |
| Funding Source | 0.21 | |||
| Industry | 328 (56.5%) | 75 (12.9%) | 178 (30.6%) | |
| Government | 94 (59.1%) | 28 (17.6%) | 37 (23.3%) | |
| Non-profit | 142 (52%) | 35 (12.8%) | 96 (35.2%) | |
| Jointly funded | 74 (58.3%) | 15 (11.8%) | 38 (29.9%) | |
| Category of Trial | <0.001 | |||
| Arrhythmia | 110 (95.7%) | 2 (1.7%) | 3 (2.6%) | |
| Coronary artery disease | 263 (53%) | 38 (7.7%) | 195 (39.3%) | |
| General/lipids | 116 (44.1%) | 37 (14.1%) | 110 (41.8%) | |
| Heart failure | 70 (47%) | 34 (22.8%) | 45 (30.2%) | |
| Vascular | 118 (58.7%) | 54 (26.9%) | 29 (14.4%) | |
| Region | <0.001 | |||
| North America | 130 (46.8%) | 61 (21.9%) | 87 (31.3%) | |
| Western Europe | 189 (50.3%) | 40 (10.6%) | 147 (39.1%) | |
| Rest-of-world | 37 (46.8%) | 10 (12.7%) | 32 (40.5%) | |
| Multiregional | 227 (76.4%) | 26 (8.4%) | 45 (15.2%) | |
| Multinational | 356 (68.3%) | 51 (9.8%) | 114 (21.9%) | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


