Mitral repair operations for correction of pure mitral regurgitation (MR) are generally quite successful. Occasionally, however, the reparative procedure incompletely corrects the MR or the MR recurs. From March 1993 to January 2016, twenty nine patients had mitral valve replacement after the initial mitral repair operation, and observations in them were analyzed. All 29 patients at the repair operation had an annular ring inserted and later (<1 year in 6 and >1 year in 21) mitral valve replacement. The cause of the MR before the repair operation appears to have been prolapse in 16 patients (55%), secondary (functional) in 12 (41%) (ischemic in 5), and infective endocarditis which healed in 1 (3%). At the replacement operation the excised anterior mitral leaflet was thickened in all 29 patients. Some degree of stenosis appeared to have been present in 16 of the 29 patients before the replacement operation, although only 10 had an echocardiographic or hemodynamic recording of a transvalvular gradient; at least 11 patients had restricted motion of the posterior mitral leaflet; 10, ring dehiscence; 2, severe hemolysis; and 2, left ventricular outflow obstruction. In conclusion, there are multiple reasons for valve replacement after earlier mitral repair. Uniformly, at the time of the replacement, the mitral leaflets were thickened by fibrous tissue. Measurement of the area enclosed by the 360° rings and study of the excised leaflet suggest that the ring itself may have contributed to the leaflet scarring and development of some transmitral stenosis.
Although the results of mitral valve repair operations performed in major medical centers by very experienced cardiac surgeons are usually excellent, an unfortunate consequence of mitral valve repair with ring insertion in a few patients is inadequate elimination of the mitral regurgitation (MR) or recurrence of mitral valve dysfunction after repair with ring insertion. From 1994 through 2015 (23 years) 32 patients who earlier had had mitral valve repair with ring insertion underwent a second mitral valve operation. This report focuses on the 29 patients in whom the second operation was mitral valve replacement.
Methods
Since March 1993, all cardiac specimens excised at cardiac operations at Baylor University Medical Center (BUMC) have been described by one of us (WCR) and most have been photographed (mainly by JMK). During this 23-year period, 32 specimens of mitral annular rings with or without the anterior mitral leaflets were submitted to the surgical pathology laboratory of the Department of Pathology of BUMC. Three of them had a second mitral repair operation and they were eliminated from this study. The remaining 29 patients all had mitral valve replacement after the initial mitral repair operation and they form the basis of this study. After examining the operatively excised annular ring and anterior mitral leaflet and chordae, the clinical records were sought and examined. The interior of the complete rings was calculated by the formula for an ellipse, which is half the length of its major axis times half the length of its minor axis times pi (3.14).
The mitral valve replacement operations in the 29 patients were performed by 11 different surgeons: 10 by 1 surgeon; 6 by another; 4 by another; 2 by another, and 1 by each of 7 different surgeons. Of the mitral valve replacement surgeons, 10 had previously done the mitral valve repair operation.
The BUMC Institutional Review Board approved this study.
Results
Certain clinical and morphologic findings are tabulated for each of the 29 patients in Table 1 . The ages of the patients at the time of the mitral valve repair operation ranged from 21 to 70 years (mean 55), and at mitral valve replacement operation, from 32 to 80 years (mean 61). The interval between the 2 operations was <1 year in 8 patients and between 1 and 24 years (mean 8) in the remaining 21 patients. The cause of the MR before the valve repair operation was prolapse in 16 patients (55%); secondary (functional) in 12 (41%), 5 of whom had coronary heart disease, and infective endocarditis, which had healed, in 1 (3%). Twelve patients (41%) had coronary artery bypass grafting, 11 at the time of the mitral repair: 6 (38%) of the 16 with mitral prolapse, and 5 (42%) of the 12 with secondary (functional) MR, and also in the 1 patient with healed infective endocarditis. Five patients had aortic valve replacement (for stenosis in 4) at the time of either the repair or replacement operation.
| Patient | Sex | Cause of MR | AF | SH | BMI (Kg/m 2 ) | Age (yrs) at MV Repair and Ring Insertion | Age (yrs) at MVR and Ring Removal | Interval between Operations | MV Ring Size (mm) and type | MDG LA-LV (mmHg) | Explanted Ring Area (cm 2 ) | Valve Type | CABG | AVR | Reason for MV Replacement | Figure Number | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | RD | Leaflet Repair Breakdown | H | Restricted PML | LVOTO | ||||||||||||||||
| 1 | Man | Prolapse | 0 | 0 | 20 | 49 | 49 | 16 days | 33 Duran | 3.9 | M | + | 0 | 0 | 0 | + | 0 | 0 | 0 | ||
| 2 | Man | Prolapse | + | + | 34 | 65 | 65 | 21 days | 30 Edwards-physio II | 5 | 3.9 (2.7 ∗ ) | M | 0 | 0 | + | 0 | 0 | 0 | + | + † | 1 |
| 3 | Man | Secondary (CAD) | 0 | + | 28 | 58 | 58 | 53 days | – | 12 | 3.3 | M | + | 0 | + | 0 | 0 | 0 | 0 | 0 | 2 |
| 4 | Woman | Healed IE | 0 | + | 43 | 68 | 68 | 63 days | 28 miral flex | – | – | B | 0 | 0 | + | + | 0 | + | 0 | 0 | |
| 5 | Man | Secondary (CAD) | 0 | + | 24 | 70 | 70 ‡ | 80 days | 28 Carbomedics | 4 | – | B | + | 0 | + | + | 0 | 0 | 0 | 0 | 3 |
| 6 | Man | Prolapse | 0 | 0 | 21 | 43 | 43 | 91 days | 33 Duran | – | 5.9 | M | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 2 |
| 7 | Man | Prolapse | 0 | + | 27 | 69 | 69 | 161 days | 33 Duran | – | – | M | + | 0 | 0 | 0 | 0 | + | 0 | 0 | 5 |
| 8 | Man | Prolapse | 0 | 0 | 28 | 55 | 55 | 237 days | – | – | 2.4 | M | 0 | 0 | 0 | + | + | 0 | 0 | 0 | |
| 9 | Woman | Secondary (CAD) | 0 | + | 29 | 57 | 58 | 1 year | – | – | 2.3 | M | + | 0 | 0 | 0 | + | 0 | 0 | 0 | 6 |
| 10 | Man | Secondary | 0 | + | 36 | 62 | 63 | 1 year | 38 ATS simulus semirigid | – | – | M | 0 | 0 | 0 | + | + | 0 | + | 0 | |
| 11 | Woman | Prolapse | 0 | + | 44 | 30 † | 32 | 2 years | 32 Carpentier Edwards | 7 | 3.5 | M | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 7 |
| 12 | Woman | Secondary | 0 | + | 22 | 45 | 47 | 2 years | 26 Cosgrove-Edwards | – | 0.3 | M | 0 | + | + | + | 0 | 0 | + | 0 | |
| 13 | Man | Prolapse | 0 | + | “obese” | 67 | 69 | 2 years | – | – | – | B | + | 0 | 0 | + | 0 | 0 | 0 | 0 | |
| 14 | Woman | Secondary § | 0 | + | 29 | 63 | 66 | 4 years | 26 Carpentier Edwards | – | 2.4 | M | 0 | 0 | + | + | 0 | 0 | + | 0 | 8 |
| 15 | Woman | Prolapse | + | + | 35 | 57 | 61 | 4 years | 30 Carpentier Edwards | 8 | 3.2 | M | 0 | 0 | + | 0 | 0 | 0 | + | 0 | 9 |
| 16 | Woman | Secondary | + | + | 54 | 55 | 60 | 5 years | 26 Carpentier Edwards | 21 | – (1.0 ∗ ) | B | 0 | + ¶ | + | 0 | 0 | 0 | + | 0 | 10 |
| 17 | Woman | Secondary (CAD) | + | + | 25 | 62 | 67 | 5 years | – | 52 | 0.3 | B | + | 0 | + | 0 | 0 | 0 | 0 | + † | 11 |
| 18 | Woman | Secondary (CAD) | + | + | 16 | 62 | 67 ∗ | 5 years | – | – | 3.1 | B | + | 0 | + | 0 | 0 | 0 | + | 0 | 12 |
| 19 | Woman | Secondary (CAD) | + | + | 25 | 66 | 72 | 6 years | Duran | – | 0.9 | M | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 13 |
| 20 | Man | Prolapse | 0 | 0 | 29 | 66 | 74 | 8 years | Carpentier Edwards | – | – | M | + | 0 | 0 | 0 | + | 0 | 0 | 0 | 14 |
| 21 | Man | Prolapse | + | + | 26 | 57 | 66 | 9 years | – | – | 4.5 | M | + | 0 | 0 | 0 | + ‖ | 0 | + | 0 | 15 |
| 22 | Woman | Secondary | 0 | + | 34 | 29 | 39 | 10 years | – | 13 | 3.9 (1.0 ∗ ) | M | 0 | + | + | + | 0 | 0 | + | 0 | 16 |
| 23 | Man | Secondary | 0 | + | 22 | 50 | 61 | 11 years | Carpentier Edwards | – | 4.5 | M | 0 | + | 0 | 0 | 0 | 0 | + | 0 | 17 |
| 24 | Man | Prolapse | 0 | + | 32 | 53 | 64 | 11 years | 30 | – | 6.4 | M | + | + | 0 | + | + | 0 | + | 0 | 18 |
| 25 | Man | Prolapse | 0 | + | 24 | 57 | 68 | 11 years | – | 14 | – | M | 0 | 0 | + | 0 | + | 0 | 0 | 0 | |
| 26 | Man | Prolapse | + | + | 21 | 57 | 70 | 13 years | – | – | 6.5 | B | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 19 |
| 27 | Woman | Prolapse | + | + | 19 | 65 | 80 | 15 years | 34 Carpentier Edwards | – | – | B | + | 0 | + | 0 | 0 | 0 | 0 | 0 | |
| 28 | Woman | Prolapse | 0 | + | 27 | 21 | 43 | 22 years | – | – | – | M | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | |
| 29 | Man | Prolapse | + | + | 28 | 47 | 71 | 24 years | – | 12 | 3.4 | B | 0 | 0 | + | 0 | 0 | 0 | + | 0 | 20 |
| Totals | 8 | 18 | 12 | 5 | 16 | 10 | 10 | 2 | 12 | 2 | |||||||||||
∗ Area of ring calculated from echocardiogram.
† LV-aortic peak gradient at rest in case #2 was 46 mm Hg increasing to 85 mm Hg with Valsalva with severe systolic anterior motion of the anterior mitral leaflet. The LV-aortic peak systolic gradient at rest in case #17 was 41 mm Hg at rest.
‡ Left ventricular aneurysm also resected.
§ Previous acute lymphocytic leukemia treated by chemotherapy with subsequent dilated cardiomyopathy and 3+/4+ mitral regurgitation.
¶ Aortic valve dysfunction probably secondary to systemic lupus erythmatosis.
‖ An Alfiere stitch had dehised. Of the 13 patients in whom the MR was of functional or ischemic origin or secondary to cardiomyopathy or to infective endocarditis that had healed, only a ring was inserted in 12 (92%).
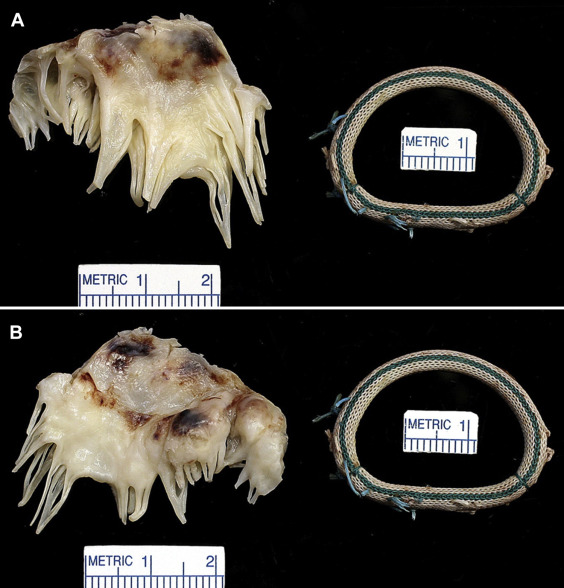
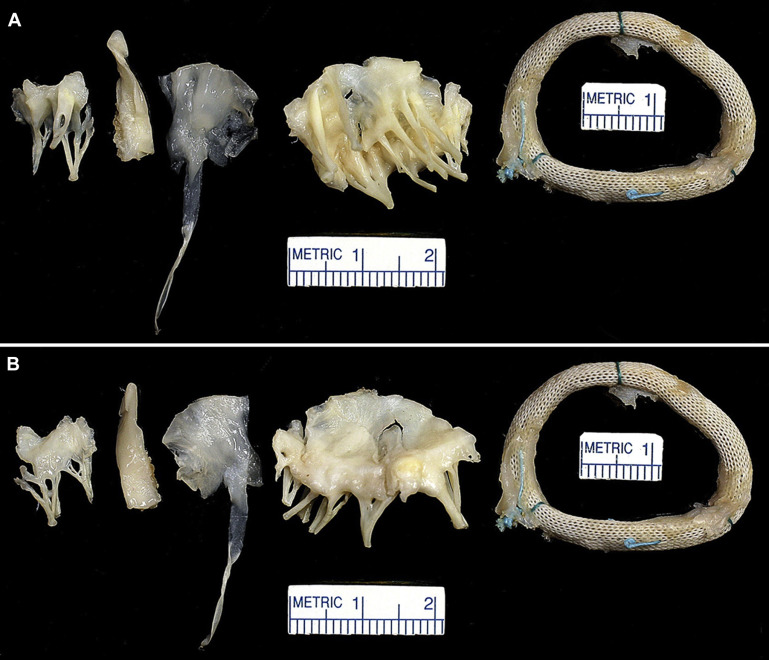
Examination of the operatively excised mitral leaflet (usually only the anterior one) in all 29 patients disclosed the leaflet to the thickened, at least focally, by fibrous tissue. The areas enclosed by the 360° annular rings (calculated in 19 patients) ranged from 2.4 to 6.4 cm 2 (mean 4.4 cm 2 ) in the 9 patients in whom the MR was the result of prolapse, and from 0.3 to 4.5 cm 2 (mean 2.3 cm 2 ) in the 9 patients with secondary MR. Either echocardiographic or hemodynamic mean left atrial to left ventricular diastolic gradients were measured in 5 of the 16 patients with prolapse and they ranged from 5 to 14 mm Hg (mean 9.2), and in 5 of the 12 patients with secondary MR, from 4 to 52 mm Hg (mean 20). In 2 patients (cases #2 and #22) the mitral area just before the replacement operation was measured by echocardiogram and also by measurement of the interior of the ring after its operative excision. In both cases the mitral area determined by echocardiogram was much smaller than that determined by morphologic measurement: 2.7 and 3.9 cm 2 , respectively, in case #2, and 1.0 and 3.9 cm 2 , respectively, in case #22.
Although MR, usually severe, was present immediately before the replacement operation in all 29 patients, some degree of mitral stenosis appeared to be present in 16 of the 29 patients, although confirmed physiologically in only 10 patients. The exact site of the stenosis was not easily determined from examination of the operatively excised leaflet and ring. Some rings appeared small: at least 2 had considerable “pannus” growing into the lumen of the ring, and the leaflets in all patients were thickened at the time of the replacement operation.
Morphologic features of the operatively excised mitral leaflets and/or rings are shown in Figures 1 to 20 . A mechanical prosthesis was employed in 20 patients (69%) and a bioprosthesis in 9 patients (31%).