Post–Myocardial Infarction Management
Deepak L. Bhatt
L. Kristin Newby
Overview
The management of acute myocardial infarction (MI) has been revolutionized in the past decade. Advances in pharmacologic and mechanical reperfusion therapy have improved the survival of patients who experience ST-segment elevation MI, and as more patients survive the initial myocardial insult, subsequent medical care has increased in importance. An appreciation of pathologic left ventricular (LV) remodeling after MI has permitted the study of medications that preserve LV function. The role of plaque stabilization with lipid-lowering therapy has been established, and the use of antiplatelet therapy to prevent recurrent atherothrombotic events continues to grow. Appropriate control of risk factors such as obesity, metabolic syndrome, diabetes, and hypertension remains prominent. Additionally, inflammation has been identified as having a significant role in the pathogenesis of ischemic events. Controversy over noninvasive risk stratification versus the need for invasive assessment continues, although the pendulum seems to be swinging toward aggressive management. Despite impressive technological advances, lifestyle modification remains a key component of secondary prevention after MI.
Glossary
Acute coronary syndrome
Plaque rupture leading to various degrees of coronary artery thrombosis and occlusion along with distal platelet microembolism. Most often refers to unstable angina and non–ST-segment elevation MI, although sometimes meant also to include ST-segment elevation MI.
Non–Q-wave myocardial infarction
Older nomenclature for MI believed to be caused by nonocclusive coronary arterial thrombus. Now referred to as non–ST-segment elevation MI.
Q-wave myocardial infarction
Older term for MI meant to imply transmural myocardial necrosis.
Remodeling
The process of LV cellular and geometric adaptation to compensate for damage sustained during MI.
ST-segment elevation myocardial infarction
MI resulting from coronary arterial occlusion by thrombus, manifested on the surface electrocardiogram as ST-segment elevation.
General Principles
Historical Perspective
Because of the use of fibrinolytic therapy and, in communities where it is available, primary percutaneous coronary intervention (PCI), the past decade has seen dramatic decreases in deaths resulting from MI. However, in the Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries III (GUSTO III) trial of acute MI, in which two different fibrinolytic regimens were tested, the 1-year mortality rate was still 11% (1), and mortality rates in the Hirulog and Early Reperfusion or Occlusion (HERO) trial were even higher (10.8% at 30 days), reflecting global differences in outcomes after acute
MI (2). Further, the additional 4% mortality rate observed in GUSTO III between 30 days and 1 year illustrates the importance of initiating secondary prevention efforts during a patient’s hospitalization for acute MI. Mortality rates in unselected populations included in MI registries are even higher: 9.4% in-hospital mortality in the National Registry of Myocardial Infarction (NRMI) (3). Furthermore, mortality rates in the United States Medicare database are more than twofold higher than mortality rates that have been observed in clinical trials. Therefore, despite improved therapies for acute MI, risk stratification, and secondary prevention are critically important in improving the outcomes of patients with acute MI by decreasing the likelihood of recurrent ischemic events, in both the short term and the long term.
MI (2). Further, the additional 4% mortality rate observed in GUSTO III between 30 days and 1 year illustrates the importance of initiating secondary prevention efforts during a patient’s hospitalization for acute MI. Mortality rates in unselected populations included in MI registries are even higher: 9.4% in-hospital mortality in the National Registry of Myocardial Infarction (NRMI) (3). Furthermore, mortality rates in the United States Medicare database are more than twofold higher than mortality rates that have been observed in clinical trials. Therefore, despite improved therapies for acute MI, risk stratification, and secondary prevention are critically important in improving the outcomes of patients with acute MI by decreasing the likelihood of recurrent ischemic events, in both the short term and the long term.
Pathophysiology
An appreciation of the pathophysiology of acute MI is necessary to understand how specific therapies may be used to target different components of the cascade of events that lead to acute MI. Plaque rupture, arterial thrombosis, myocardial necrosis, and adverse LV remodeling are parts of the continuum that leads to symptomatic manifestations and long-term sequelae of acute MI.
Plaque Rupture and Thrombosis
The inciting event of acute MI is plaque rupture. Numerous factors, which are only partially understood, determine the occurrence of plaque rupture in a susceptible individual. Production of matrix metalloproteinases leads to degradation of the shoulder of a coronary plaque, resulting in exposure of its lipid-rich core to flowing blood (4,5,6). This triggers formation of platelet-rich thrombus. When the degree of thrombus is flow occlusive or platelet microemboli occlude distal flow, ischemia occurs, and myocardial stunning and necrosis follow.
The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) may exert part of their benefit by decreasing the lipid content of plaques, rendering them less prone to rupture (7,8). Antithrombotic therapy decreases the tendency for occlusive thrombus formation; in particular, antiplatelet therapy decreases platelet aggregation at the site of ruptured plaque. Thus, in concert, statin therapy and antiplatelet therapy decrease the chance of MI occurring.
Left Ventricular Remodeling
When occlusive thrombus has developed and caused ischemia for as little as 45 to 60 minutes, myocardium is irreversibly damaged. When this necrosis is transmural, the left ventricle may undergo a process called remodeling. This adaptation occurs at both the cellular level (destruction of connective tissue with slippage of myofilaments) and the whole-organ level (changes in the size and shape of the left ventricle) and results from adaptive (or maladaptive) mechanisms, such as apoptosis and fibroblast and myocyte hypertrophy triggered by increased wall stress from the inciting cellular changes (9). Angiotensin-converting enzyme (ACE) inhibitors greatly decrease this process of adverse remodeling, and it appears that angiotensin receptor blockers (ARBs) share this property. This explains, at least in part, the marked benefits of ACE inhibition or angiotensin receptor blockade among patients who have ventricular dysfunction after MI.
Arrhythmogenic Substrate
The zone of myocardium surrounding the infarcted area is particularly prone to electrical instability. This often manifests as premature ventricular contractions (PVCs) and predisposes the patient to ventricular arrhythmia. β-Blockers are useful in reducing the electrical irritability of the damaged myocardium. Implantable cardioverter-defibrillators (ICDs) are the best line of defense in treating electrical excitability that causes potentially lethal arrhythmia, particularly in the setting of impaired LV function.
Open-Artery Hypothesis
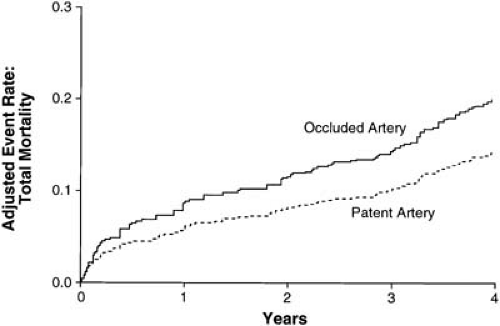
Even if the LV muscle supplied by an occluded artery is dead, revascularization may still favorably effect ventricular remodeling and promote electrical stability. Depending on the duration of ischemia, among other factors, the myocardial cells either die or go into a state of hibernation. In this latter stage, revascularization can restore contractile functionality to what otherwise appears to be dead myocardium. Thus, there are a number of reasons, both established and theoretical, why vessel patency can improve prognosis (Fig. 21.1) (10).
Clinical Management
Exercise stress testing, noninvasive imaging, and coronary angiography are all used to risk stratify patients and determine their subsequent management. Risk stratification models may help to determine the most appropriate in-hospital and long-term management of patients with acute MI (11).
Noninvasive Assessment of Left Ventricular Function and Reperfusion
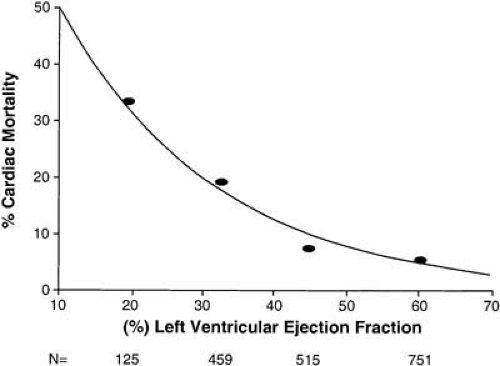
Transthoracic echocardiography is a standard technique used not only to determine the presence of mechanical complications of MI, but also to provide an estimate of LV systolic and diastolic function. Indeed, at least on a population level, LV ejection fraction has been shown to be a powerful predictor of outcome (Fig. 21.2) (12). Another alternative for post-MI assessment of LV systolic function that also allows an assessment of infarct size and remaining myocardial viability is cardiac magnetic resonance imaging (MRI). Cardiac MRI has been used successfully to predict recovery of systolic function at 6 months (13). A number of new modalities are being investigated as aids for
determination of tissue level reperfusion after MI, but none are widely available for routine use. Contrast echocardiography is one such modality (14,15), and in conjunction with ST-segment resolution may be useful in gauging reperfusion (16). MRI may be even more sensitive than contrast echocardiography in detection of microvascular obstruction (17), and such modalities may ultimately be important adjuncts to more conventional determinants of infarct size (18). These newer modalities may refine assessment of LV function so that an evaluation of microvascular integrity will become part of analyzing the state of the infarcted myocardium, at both a whole-organ and a cellular level.
determination of tissue level reperfusion after MI, but none are widely available for routine use. Contrast echocardiography is one such modality (14,15), and in conjunction with ST-segment resolution may be useful in gauging reperfusion (16). MRI may be even more sensitive than contrast echocardiography in detection of microvascular obstruction (17), and such modalities may ultimately be important adjuncts to more conventional determinants of infarct size (18). These newer modalities may refine assessment of LV function so that an evaluation of microvascular integrity will become part of analyzing the state of the infarcted myocardium, at both a whole-organ and a cellular level.
Stress Testing
The role of stress testing in the management of patients after MI has been extensively studied. The value of exercise stress testing depends greatly on whether the patient has undergone revascularization (19). Guidelines have been developed to aid in the appropriate use of stress testing (20). Data from NRMI show that predischarge stress testing currently is performed in fewer than 10% of American patients, with the majority undergoing in-hospital revascularization or outpatient stress testing (3).
Certain features on a stress test identify patients at high risk of recurrent ischemic events. LV dilatation, pulmonary uptake of thallium-201, ejection fraction less than 40%, and reversible defects in the territory of the infarction are all markers of high risk on postexercise nuclear imaging scans. Additionally, large fixed defects and multivessel disease considerably raise the risk profile of the patient. Normal or low-risk results of a dipyridamole stress test predict an annual rate of death, MI, or urgent revascularization of less than 2%.
The Danish Trial in Acute Myocardial Infarction (DANAMI) randomly assigned 1,008 patients who had received fibrinolysis for MI and had inducible ischemia on stress testing to receive either invasive or conservative treatment (21). Those patients assigned to the invasive arm had a statistically significant reduction in the primary end points of death, MI, or readmission for unstable angina. The authors appropriately concluded that patients who have inducible ischemia detected before discharge should undergo coronary angiography.
Exercise stress testing can provide valuable information, primarily because the inability to exercise confers a poor prognosis (22,23). Although rare, myocardial rupture is possible during exercise stress testing or dobutamine stress testing in the post-MI period (24). As an alternative to submaximal exercise stress testing before hospital discharge, pharmacologic stress testing with dipyridamole 2 to 4 days after MI may be performed safely (25). In one study, dipyridamole stress testing outperformed submaximal exercise testing with nuclear imaging in its ability to predict postdischarge cardiac events (25).
Coronary Angiography
As noted, the DANAMI study showed that patients with inducible ischemia after acute MI had better outcomes if they were treated with an invasive strategy as opposed to a conservative one (21). Revascularization, either percutaneously or surgically, was found to be beneficial. An analysis of the patients from the DANAMI study who underwent coronary artery bypass grafting (CABG) was performed (26). The median time to CABG after acute MI was approximately 6 weeks. Patients who had inducible ischemia, including silent ischemia, after acute MI benefited from CABG.
Similarly, spontaneous ischemia in the form of postinfarction angina is a serious development that warrants invasive investigation. Armstrong et al. (27) reported on the incidence and consequences of recurrent ischemia across the spectrum of ST-segment elevation MI and non–ST-segment elevation acute coronary syndrome (ACS) in an analysis from the GUSTO-IIb trial database (27). In this cohort, 35% of patients with non–ST-segment elevation ACS had recurrent ischemia compared with 23% of patients with ST-segment elevation MI. Particularly when recurrent ischemia was classified as refractory, both 30-day and 1-year survivals were substantially lower than if no recurrent ischemia occurred. Similarly, the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico 3 (GISSI-3) trial analyzed the outcomes of patients with early angina after MI who were managed conservatively (28). Patients with post-MI angina had more in-hospital reinfarction, but despite successful medical management while in the hospital in many cases, these patients had a higher rate of death or reinfarction at 6 months. In fact, the presence of early post-MI angina was an independent predictor of the 6-month reinfarction or death. Among patients with early angina, the rate of reinfarction was 12%, versus 5% among patients without early angina (P <.0001). The rate of death was also higher among patients with early angina than in those without early angina (13% versus 7%, respectively; P <.0001). Based on these observations, angiography for ischemic symptoms after MI is given a class I indication in the American College of Cardiology/American Heart Association guidelines for coronary arteriography (29).
Indirect evidence from comparative studies favors an invasive approach to treatment of MI. Compared with Canadian patients, American patients are more likely to undergo catheterization and angioplasty. This difference in approach appears to lead to better outcomes in American patients (30). A study comparing outcomes after MI in areas in France and Spain with different rates of angiography reached a similar conclusion regarding the superiority of an invasive approach (31).
However, older, randomized studies such as the Treatment of Post-Thrombolytic Stenoses trial do not favor routine angiography after successful fibrinolysis, even when angiography demonstrates a “significant” stenosis (32). The Should We Intervene following Thrombolysis trial did not find a benefit for routine angiography after fibrinolysis either (33). Likewise, the Thrombolysis in Myocardial Infarction (TIMI) II-B and European Cooperative Study Group trials were unable to demonstrate an advantage to a routine invasive approach (34,35). The
Thrombolysis and Angioplasty in Myocardial Infarction study found no advantage to immediate angioplasty after fibrinolysis in comparison with delayed angioplasty 5 to 10 days later (36). Perhaps the prothrombotic effects at the site of ruptured plaque that systemic fibrinolysis can create, coupled with further arterial trauma from balloon angioplasty, led to lack of favorable outcomes with invasive therapy (37). Therefore, the results of these trials may no longer apply in an era marked by greater use of stents and IV glycoprotein IIb/IIIa inhibition, but this issue needs to be examined prospectively (38). Recently, the ASSENT IV trial results were presented. This trial showed an increased risk of ischemic events and also major bleeding in patients who received a full dose of fibrinolysis followed by PCI versus primary PCI without antecedent lytic use (39). Therefore, in patients who appear to have successfully reperfused after fibrinolysis, or those who have received no reperfusion therapy acutely, it is still reasonable to use noninvasive risk stratification before invasive therapy, with its attendant risk.
Thrombolysis and Angioplasty in Myocardial Infarction study found no advantage to immediate angioplasty after fibrinolysis in comparison with delayed angioplasty 5 to 10 days later (36). Perhaps the prothrombotic effects at the site of ruptured plaque that systemic fibrinolysis can create, coupled with further arterial trauma from balloon angioplasty, led to lack of favorable outcomes with invasive therapy (37). Therefore, the results of these trials may no longer apply in an era marked by greater use of stents and IV glycoprotein IIb/IIIa inhibition, but this issue needs to be examined prospectively (38). Recently, the ASSENT IV trial results were presented. This trial showed an increased risk of ischemic events and also major bleeding in patients who received a full dose of fibrinolysis followed by PCI versus primary PCI without antecedent lytic use (39). Therefore, in patients who appear to have successfully reperfused after fibrinolysis, or those who have received no reperfusion therapy acutely, it is still reasonable to use noninvasive risk stratification before invasive therapy, with its attendant risk.
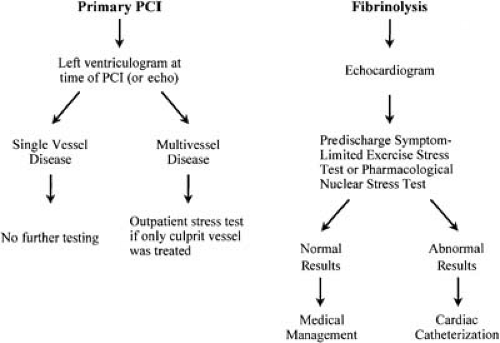 FIGURE 21.3. Risk assessment algorithm for the post–MI patient, depending on the primary mode of reperfusion. Abbreviations: echo, echocardiogram; PCI, percutaneous coronary intervention. |
One of the benefits of an invasive strategy is that it can provide diagnostic information and definitive therapy in the form of PCI. Whereas Fragmin and Fast Revascularisation during Instability in Coronary Artery Disease II (FRISC-II) and Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy were studies demonstrating the superiority of an invasive approach over a conservative strategy in non–ST-segment elevation ACSs, it may prove to be legitimate to extrapolate these results to the management of patients after acute ST-segment elevation MI (40,41,42,43,44,45).
In the future, ST-segment resolution after fibrinolytic therapy may be used to guide the need for invasive therapy. Contrast echocardiography or perfusion MRI may also prove to have clinical usefulness in assessing lack of tissue-level perfusion and the need for invasive therapy. An algorithm for risk stratification is shown in Figure 21.3.
Assessment of Inflammatory Activity
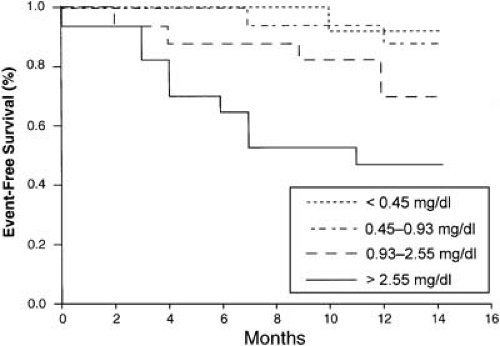
Although an assessment of myocardial necrosis and microvascular obstruction is useful, measures of heightened inflammation, such as an elevated C-reactive protein (CRP) level, may provide additional ability to risk stratify patients (46,47). The value of CRP levels was examined in a study of 64 patients with an uncomplicated MI (48). All patients had normal LV function with no evidence of ischemia on a predischarge ergometer test. With each successive increase in quartile of CRP, the risk of cardiac death, recurrent MI, or new-onset angina in the ensuing year increased (Fig. 21.4). Even cruder indices of inflammation, such as the white blood cell (WBC) count, appear to help to risk stratify patients (49). A study of 975 patients with acute MI found that those who had angiographic thrombus were more likely to have an elevated WBC count, perhaps illustrating the interplay between inflammation and thrombosis (49). Furthermore, increasing elevation in levels of WBCs was associated with higher mortality.
Beyond their role in risk stratification, markers of inflammation may help to gauge the incremental benefit of invasive therapy, in a manner complementary to troponin elevation. This paradigm has been shown to be true for non–ST-segment elevation ACSs in two separate analyses, the results of which may apply to the acute MI setting. In an analysis of almost 12,000 patients with ACS, elevated WBC count correlated with 6-month mortality (50). However, in those patients undergoing in-hospital revascularization, the deleterious effect of an elevated WBC count were substantially lessened (50). Similarly, in FRISC-II, an invasive strategy (compared with a conservative approach) was seen to be of particular benefit in patients with elevated CRP, troponin T, or interleukin-6 levels (51).
Risk of Arrhythmia
The role of routine telemetry in patients after MI is well established. In fact, perhaps the major advance of the cardiac intensive care unit was the development of continuous monitoring for ventricular arrhythmia, allowing prompt defibrillation. High-grade heart block may develop after anterior wall MI, and permanent pacemaker implantation may be necessary. Atrial fibrillation may complicate MI, often as a result of concomitant ventricular dysfunction from a large infarction.
PVCs after MI are commonly observed on telemetry, and an increased frequency definitely increases the risk of subsequent arrhythmic death (52). Therefore, it was logical to think that decreasing or eliminating PVCs would be desirable. However, the Cardiac Arrhythmia Suppression Trial (CAST) I and CAST II showed that routine suppression of PVCs was not beneficial and that suppression with the agents studied was in fact associated with increased mortality (53,54,55). Therefore, routine suppression of asymptomatic ventricular arrhythmias was abandoned.
An ICD may be indicated to prevent life-threatening arrhythmia in certain high-risk patients after acute MI, particularly those with prior life-threatening ventricular arrhythmias or LV systolic dysfunction. The randomized clinical trials that have explored the use of ICD therapy were recently summarized by Al-Khatib et al. (56). In patients with prior ventricular fibrillation or symptomatic sustained ventricular tachycardia, the Antiarrhythmics versus Implantable Defibrillators Trial demonstrated the superiority of ICD over antiarrhythmic therapy, with a 3-year mortality rate of 24.6% versus 35.9%, respectively (57). Several trials have also randomized high-risk patients (defined clinically and/or guided by electrophysiologic testing) who were without prior life-threatening arrhythmias to prophylactic ICD therapy. All but two of these trials found a mortality benefit among patients receiving ICD therapy (57,58,59,60,61,62,63,64). In CABG-PATCH there was no mortality benefit from prophylactic ICD implantation at the time of CABG. The Defibrillator in Acute Myocardial Infarction Trial (DINAMIT), the other trial to show no benefit from ICD therapy, was the only trial to test prophylactic ICD implantation in the context of recent acute MI, and suggested that patients should not receive an ICD within one month of an MI (63). Interestingly, in a subanalysis of 14,609 patients from the VALIANT trial, which randomized acute MI patients with clinical evidence of heart failure or LV ejection fraction 40% or less to captopril, valsartan, or the combination, 7% died suddenly or had a resuscitated cardiac arrest within 6 months of their index MI, and 19% of all such events during the median 24.7 months of follow-up and 83% of all sudden deaths occurred within the first 30 days (65).
Thus, given that it is a very high-risk period for sudden death or resuscitated cardiac arrest, the reason for the failure to show a benefit of ICD implantation within 6 to 40 days of acute MI in the DINAMIT trial is unclear, but other competing factors may explain the overall mortality results observed in the trial. At present, recommendations are for prophylactic ICD implantation 1 month or more after MI in patients with an EF of less than 30% at that time, and many electrophysiologists wait at least 3 months and reassess LV function before deciding about prophylactic ICD implantation.
Concerns about the health economic implications of prophylactic ICD implantation in the hundreds of thousands of patients who would qualify for such therapy based on the results of these clinical trials were recently addressed in a study of the cost effectiveness of ICD therapy by Sanders et al. (66). Based on the results of six trials showing a mortality benefit from prophylactic ICD implantation in high-risk patients with reduced ejection fraction, it was estimated that prophylactic placement of an ICD would increase life expectancy by 2.12 to 6.21 years. Depending on the patient population, as defined by the individual trials’ inclusion criteria, the cost effectiveness of prophylactic implantation was between $34,000 and $70,200 per quality-adjusted life-year and remained under $100,000 per quality-adjusted life-year across a broad range of sensitivity analyses. Thus, the use of ICD therapy in selected patient populations appears to have cost effectiveness in the range of other standard therapies for coronary artery disease. However, whereas ICD implantation definitely has a favorable impact on mortality rates in populations of patients and has been shown to be cost effective, the feasibility of such an approach, especially if it were applied broadly to all MI patients meeting trial criteria, remains to be determined. Methods to further define which patients are at highest risk and will receive most benefit from ICD therapy are needed. Home automatic external defibrillators are being evaluated in an ongoing randomized trial in patients with an anterior MI in the past month, a period of time that has been identified as being particularly high-risk for sudden cardiac death (65).
Medical Therapy
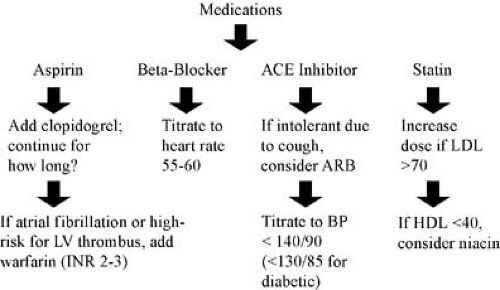
Several classes of medications designed to improve outcomes after acute MI have undergone rigorous evaluation in large, randomized, controlled trials. It is imperative that physicians screen post-MI patients for criteria that indicate that specific medications should be prescribed (Fig. 21.5).
Afterload Reduction
Angiotensin-Converting Enzyme Inhibitors
Use of ACE inhibitors after acute MI is supported by a vast amount of evidence (Table 21.1) (67,68,69). The Acute Infarction Ramipril Efficacy study found that ramipril decreased mortality after MI among patients with heart failure (70), and the Survival and Ventricular Enlargement trial demonstrated the significant mortality benefit from administration of captopril after MI among patients with asymptomatic LV dysfunction (71). The Trandolapril Cardiac Evaluation study showed that the benefit of ACE inhibition persisted through 2 years of therapy, suggesting that ACE inhibition should be continued indefinitely (72). In fact, the results of the Heart Outcomes Prevention Evaluation (HOPE) study suggest that ACE inhibitors may be indicated in all patients who have had an MI (73). Further, the EUROPA trial also found a reduction in cardiovascular events in patients with stable coronary artery disease without LV dysfunction who were randomized to the ACE inhibitor perindopril versus placebo (74). However, the PEACE trial did not find a significant benefit of ACE inhibition with trandolapril versus placebo in patients with stable heart disease; this may reflect that the patients enrolled in this trial were lower risk than in the prior two trials (75).
Early initiation of oral ACE inhibitors is recommended for these patients, unless hypotension is present (76,77,78). However, the data do not support the early administration of IV ACE inhibitors (79). The early benefit of ACE inhibition on minimizing LV dilatation is most marked when reperfusion has not occurred, but other beneficial effects of ACE inhibitors likely apply to all patients (80). Despite these favorable data, ACE inhibitors are underprescribed after acute MI (81). Unless systolic blood pressure is lower than 100 mm Hg, ACE inhibition should generally be initiated within 24 hours of acute MI (78).
TABLE 21.1 Large Trials of the use of Ace Inhibitors after Acute MI, Demonstrating Mortality Rates For Placebo and ACE Inhibitor Arms | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Angiotensin-Receptor Blockers
At present, ARBs appear to be an acceptable alternative to ACE inhibitors, particularly for patients who are unable to tolerate ACE inhibitors because of side effects such as cough. Whether any incremental benefit occurs with the addition of an ARB to the treatment regimen for patients who are on an ACE inhibitor has been evaluated in the Valsartan in Acute Myocardial Infarction Trial, which compared the ARB valsartan, the ACE inhibitor captopril, or the combination in MI patients with heart failure (82). In this population, there was no difference in mortality between the captopril and valsartan groups, and the combination did not provide any greater benefit than either agent alone, but did increase the incidence of drug-related side effects. However, in a more broad population of patients with systolic dysfunction in the Candasartan in Heart failure Assessment of Reduction in Mortality (CHARM)-Added trial, the ARB, candasartan, added to ACE inhibitor was found to be superior to the use of ACE inhibitor alone (83). Importantly, in the CHARM-Alternative Trial, Granger et al. (84) showed that candasartan alone reduced mortality and morbidity by nearly 30% among patients with systolic dysfunction but intolerant of ACE inhibitors, supporting the use of ARBs as an alternative to ACE inhibitors in ACE inhibitor intolerant patients.
TABLE 21.2 Event Rates for Selected Large Trials of the use of β-Blockers Versus Placebo after Acute MI | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
β-Blockers
The role of β-blockers in patients who have experienced MI is well established (Table 21.2) (85). Beneficial effects result from decreases in heart rate, blood pressure, myocardial oxygen demand, and arrhythmogenesis (86). Newer evidence suggests a favorable influence on LV remodeling as well (87). In aggregate, the data suggest that β-blockade reduces nonfatal MI by approximately 25%, which is paralleled by a 25% reduction in mortality (88,89). Although these data were gathered largely before the fibrinolytic era, TIMI II-B found that even in patients who had received fibrinolysis, compared with later administration of β-blockers, early β-blockade reduced recurrent chest pain and reinfarction (90). Furthermore, early β-blockade has been associated with a lower incidence of
intracranial hemorrhage after fibrinolysis (91). Conversely, in a secondary analysis from the GUSTO-1 trial database, Pfisterer et al. (92) raised concerns about the safety and efficacy of early IV β-blockade. In their analysis, they observed that patients treated with atenolol at any point were generally at lower risk, but that their adjusted 30-day mortality was significantly lower that those not receiving atenolol. However, patients treated with early intravenous then oral atenolol versus oral treatment alone had an increased odds of mortality (1.3; 95% confidence interval, 1.0–1.5; P = .02). Patients treated with IV atenolol had more heart failure, shock, recurrent ischemia, and pacemaker use than those treated with oral atenolol, but there were no significant differences in the rates of stroke, intracranial hemorrhage, and reinfarction. More recently, the COMMIT randomized trial also suggested that very early initiation of β-blockade was deleterious among patients who have a low blood pressure at admission and may be in impending cardiogenic shock (93). In COMMIT, early initiation of metoprolol increased the risk of cardiogenic shock, although it decreased recurrent infarction. Overall, though, there was no reduction in mortality. However, once patients were stabilized, β-blockers appeared to decrease the risk of sudden cardiac death. The lesson from these studies appears to be that in high-risk patients with hypotension or signs of heart failure, it is best not to initiate β-blockade, until the patient has stabilized.
intracranial hemorrhage after fibrinolysis (91). Conversely, in a secondary analysis from the GUSTO-1 trial database, Pfisterer et al. (92) raised concerns about the safety and efficacy of early IV β-blockade. In their analysis, they observed that patients treated with atenolol at any point were generally at lower risk, but that their adjusted 30-day mortality was significantly lower that those not receiving atenolol. However, patients treated with early intravenous then oral atenolol versus oral treatment alone had an increased odds of mortality (1.3; 95% confidence interval, 1.0–1.5; P = .02). Patients treated with IV atenolol had more heart failure, shock, recurrent ischemia, and pacemaker use than those treated with oral atenolol, but there were no significant differences in the rates of stroke, intracranial hemorrhage, and reinfarction. More recently, the COMMIT randomized trial also suggested that very early initiation of β-blockade was deleterious among patients who have a low blood pressure at admission and may be in impending cardiogenic shock (93). In COMMIT, early initiation of metoprolol increased the risk of cardiogenic shock, although it decreased recurrent infarction. Overall, though, there was no reduction in mortality. However, once patients were stabilized, β-blockers appeared to decrease the risk of sudden cardiac death. The lesson from these studies appears to be that in high-risk patients with hypotension or signs of heart failure, it is best not to initiate β-blockade, until the patient has stabilized.
β-Blockers remain underused after MI, sometimes because of unfounded concerns regarding relative contraindications such as diabetes or chronic obstructive pulmonary disease (94,95,96). β-Blockers have a similar relative benefit in the presence and absence of heart failure, although the absolute benefit is magnified in those with heart failure (97). Although numerous studies have confirmed the benefit and safety of β-blockade in the elderly, underuse remains a problem (98,99,100). The optimal dose of β-blockade in elderly patients, is not known, but doses lower than those that were used in several of the early randomized trials may be just as effective (101,102).
The limited available evidence suggests that β-blockade and ACE inhibition are complementary (103,104,105,106,107,108). In patients who experience congestive heart failure after MI, both classes of medication seem to be indicated. At the other end of the spectrum, in patients with normal LV function, perhaps only one of these classes of medication is needed. Without prospective data examining the incremental value of combination therapy, other patient factors, such as history of arrhythmia or diabetes, should also be considered in deciding which of these agents to use.
Aldosterone Antagonism
The Randomized Aldactone Evaluation Study found a 30% reduction in mortality among patients with severe heart failure randomized to spironolactone (109). Subsequently, the Eplerenone Post-AMI Heart Failure Efficacy and Survival Study demonstrated a 15% reduction in mortality among patients with acute MI complicated by heart failure (110). However, without close monitoring, hyperkalemia can be a potentially lethal complication of aldosterone antagonism (111).
Calcium Channel Blockers
Given the abundance of mortality data supporting the use of ACE inhibitors and β-blockers, calcium channel blockers should not be considered first-line therapy in post-MI patients (112). The short-acting calcium channel blockers, especially nifedipine, should not be used for acute or chronic therapy, because these agents do not decrease mortality rates and appear to raise the risk of cardiac events (113,114,115,116,117). Without further supportive evidence, the main role of calcium channel blockers is for control of persistent hypertension in post-MI patients, or as primary therapy in the very rare patient with coronary artery spasm (118). The calcium channel blocker amlodipine does have strong data supporting its role as an antihypertensive and it appears to have a role in decreasing plaque regression and preventing cardiovascular events in patients with coronary artery disease without hypertension (119). Because it does not affect heart rate, it can be safely combined with a β-blocker.
Nitrates
No study has provided compelling evidence that nitroglycerin reduces mortality rates after acute MI. GISSI-3 found no mortality advantage or decrease in the incidence of LV dysfunction from the use of transdermal nitroglycerin (67). The Fourth International Study of Infarct Survival found no mortality benefit from use of an oral mononitrate preparation in any subgroup examined (68). Nevertheless, because of the acute antiischemic effect of these agents, every patient who has experienced an MI should be instructed to carry a bottle of sublingual nitroglycerin at all times and should be instructed in its safe use.
3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors
Statins are strongly indicated for use as secondary prevention after acute MI. The Cholesterol and Recurrent Events (CARE) trial demonstrated that pravastatin, compared with placebo, reduced the rate of death or MI in patients with previous MI (120). In addition to beneficial effects on reinfarction, the CARE study showed that statin use decreased the risk of stroke in survivors of acute MI (121,122). Several other trials have validated the role of statins in secondary and even primary prevention (123). Whether there are benefits specific to one statin over another remains to be elucidated. The Pravastatin or Atorvastatin Evaluation and Infection Therapy—Thrombolysis in Myocardial Infarction 22 trial randomized over 4,000 patients with ACS to receive atorvastatin 80 mg/d versus pravastatin 40 mg/d for a mean duration of 24 months and demonstrated a 16% reduction in ischemic events with a more aggressive LDL cholesterol reduction strategy with atorvastatin 80 mg (124




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree