Estimates from clinical trials and small observational studies of the incidence of pulmonary toxicity (PT) associated with amiodarone range from 1% to 10%. We report a unique study of the population-based incidence and potential predictors of PT in a real-world atrial fibrillation (AF) population. We conducted a retrospective cohort study of patients ≥65 years old discharged with AF using linked administrative databases from Quebec, Canada from 1999 to 2007. “Users” and “nonusers” of amiodarone were identified by prescriptions dispensed within 7 days after hospital discharge. PT was defined through International Classification of Diseases, Ninth Revision and Tenth Revision codes for pulmonary fibrosis, alveolar/interstitial lung disease, and adult respiratory distress syndrome. Potential risk factors for PT were identified using multivariable Cox regression. PT occurred in 250 of 6,460 amiodarone users (3.87%) and 676 of 50,993 nonusers (1.33%). Age-standardized PT incidences were 28.30 and 16.02 per 1,000 person-years in men and women users, respectively, and 14.05 and 8.82 per 1,000 person-years in nonusers, respectively. It was associated with amiodarone exposure at all doses (≤200 mg/day, hazard ratio 1.62, 1.35 to 1.96; >200 mg/day, 1.46, 1.22 to 1.75). Other predictors of PT included increasing age (1.01 per year, 1.00 to 1.02), male gender (1.37, 1.19 to 1.57), chronic obstructive pulmonary disease (2.53, 2.21 to 2.89), and renal disease (1.26, 1.06 to 1.50). In conclusion, the population-based incidence of amiodarone PT is in the lower range of what has been previously reported. However, patients with AF who use amiodarone have an approximately 50% higher risk of PT than nonusers. Clinicians may be able to use the present results to identify patients at higher risk for PT and implement strategies to increase monitoring or select alternative therapy.
Amiodarone is a highly effective antiarrhythmic. Its long 1/2 life causes long-lasting major adverse effects (pulmonary toxicity [PT], thyroid dysfunction, hepatotoxicity, and skin discoloration). Amiodarone PT, first reported in 1980, is 1 of the most serious adverse effects, thus limiting its use. The incidence of amiodarone PT is reported to be 0% to 10% (0% to 8% in randomized controlled trials, 2% to 8% in prospective cohorts and case series, and <2% in meta-analyses). Mortality is estimated at 1% to 33%. Onset of amiodarone PT is unpredictable and insidious, often remaining a diagnosis of exclusion, after consideration of heart failure, pulmonary embolism, and pulmonary infection. PT secondary to amiodarone has nonspecific diagnostics; however, impairment of diffusion capacity for carbon monoxide, total lung capacity, and forced vital capacity are common. Increased age, pre-existing lung disease, dose, and duration of therapy are potential risk factors. Although higher doses tend to be more toxic, low doses of amiodarone can cause serious toxicity. Meta-analyses have provided estimates of amiodarone PT. However, participants in clinical trials are usually healthier than the general population. Although many case series have described the nature of the presentation of amiodarone PT, the samples were small or the studies were limited to a single center. Given these limitations, there is a need to estimate the population-level incidence of and risk factors for amiodarone PT. Furthermore, as new drug and nondrug therapies for rhythm control in atrial fibrillation (AF) become available, there is a need to revisit the risks of existing therapies in a contemporary patient cohort.
Methods
We conducted a retrospective observational cohort study of patients with AF using linked administrative data. For patient identification, we used the hospital discharge abstract database Maintenance et Exploitation des Données pour l’Étude de la Clientèle Hospitalière, which contains inpatient diagnostic and therapeutic procedures codes. Medications were identified by the drug identification number in the provincial prescription claims database Régie de l’Assurance Maladie du Québec for information on medication use and duration of therapy, which is available only for those ≥65 years old. Survival data were obtained from the Maintenance et Exploitation des Données pour l’Étude de la Clientèle Hospitalière and Régie de l’Assurance Maladie du Québec databases.
Subjects were included if they were ≥65 years old and discharged with a primary or secondary diagnosis of AF from January 1, 1999 through March 31, 2007 identified according to International Classification of Diseases, Ninth Revision and Tenth Revision (ICD-9/10) codes 427.3, 427.31, and 427.32/I48. We conducted an internal validation using AF diagnoses in the hospital discharge abstract database and the physician billing database. To ensure patients had nontransient AF and for patients without a primary diagnosis of AF, 2 secondary diagnoses of AF were required. For patients with >1 AF diagnosis code, the first hospitalization discharge date was the cohort entry date. Patients were excluded if they had AF coded as a complication; a history of valvular disease/surgery; AF within 30 days of coronary artery bypass surgery, pericardial surgery, or structural cardiac repair; history of pulmonary fibrosis within 12 months; or were residents of long-term care facilities.
Exposure was determined based on amiodarone prescriptions filled within 7 days of index hospital discharge. A sensitivity analysis was conducted by extending this period to 30 days. Those without an amiodarone prescription were considered nonusers. Discontinuation of an amiodarone prescription was defined as the end date of the last filled prescription plus 60 days or end of follow-up, whichever came first. The 60-day period was used because of the long 1/2 life of amiodarone and to avoid miscoding patients who were late in refilling their prescription as unexposed. To calculate incidence, quantity, tablet strength, and days of supply of each amiodarone prescription were used to create an average daily dose (milligrams per day). Patients were categorized into low-dose (≤200 mg/day) or high-dose (>200 mg/day) groups.
Patients were followed from AF diagnosis until occurrence of the study outcome of first principal or secondary PT diagnosis (pulmonary fibrosis ICD-9/10 515/J84, J84.1, J84.8, J84.9, J70.2, J70.3, J70.4, J70.8, J70.9; idiopathic pulmonary fibrosis ICD-9 516.3; or other alveolar/interstitial disease ICD-9 516; adult respiratory distress syndrome ICD-9/10 518.5, 518.82/J80) or March 31, 2007, whichever came first. Idiopathic pulmonary fibrosis was included because PT is more likely a result of amiodarone-associated pulmonary fibrosis rather than idiopathic pulmonary fibrosis. We considered a broad definition of PT because it may be diagnosed as pulmonary fibrosis, interstitial pneumonitis, pulmonary alveolitis, or acute respiratory distress syndrome. Death was documented if it occurred before the end of the follow-up period (in the absence of the PT outcome). Follow-up for each subject was censored after the first PT diagnosis, drug discontinuation, end of follow-up, or death, whichever came first.
Potential risk factors for the development of PT that we evaluated included patient demographics (age, gender), co-morbidities within previous 12 months (chronic obstructive pulmonary disease [COPD], heart failure, diabetes mellitus, hypertension, coronary artery disease, stroke, chronic kidney disease [CKD], hypothyroidism, liver disease), AF medications (nonamiodarone antiarrhythmics, rate-control medications [β blockers, verapamil, diltiazem, digoxin]), amiodarone dose (≤200 or >200 mg/day, nonusers 0 mg), and strong cytochrome 3A4 inhibitors (erythromycin, clarithromycin, ketoconazole, itraconazole, nelfinavir, or ritonavir).
Descriptive statistics were used to compare baseline characteristics between groups. Crude and age-standardized incidence rates of PT overall and stratified by gender, amiodarone dose, and significant risk factors for PT (number per 1,000 person-years) were estimated for users and nonusers. The reference group for age standardization was all patients in the AF cohort for fiscal year 2002. Crude incidence of PT in users was calculated by dividing the number of subjects with PT by the sum of observation times of all subjects who were exposed to any amiodarone. The crude mortality rate for those who developed PT was estimated. Multivariable Cox proportional hazards models were used to estimate hazard ratios with 95% confidence intervals for outcome of PT between exposed and unexposed subjects and to identify potential predictors of PT. Sensitivity analyses included dose–gender and dose–age interaction terms and exposure based on prescriptions filled within 30 days (instead of 7 days) of index hospital discharge. All p values were 2-sided. Data were analyzed using SAS 9.1 (SAS Institute, Cary, North Carolina). This study was approved by the ethics review board of McGill University, Montreal, Quebec, Canada and Western University of Health Sciences, Pomona, California.
Results
In total 57,393 patients were included in the cohort: 6,460 users and 50,933 nonusers. Significantly more men were in the user than in the nonuser group. COPD, heart failure, diabetes, coronary artery disease, hypothyroidism, and CKD were significantly higher in the user group. Conversely, age, stroke/transient ischemic attack, and liver disease were significantly higher in nonusers. As expected, users were less likely to be receiving rate-control and nonamiodarone antiarrhythmic medications than nonusers ( Table 1 ).
| Variable | Amiodarone User | p Value | |
|---|---|---|---|
| Yes | No | ||
| (n = 6,460) | (n = 50,933) | ||
| Mean age (years) | 77.5% | 79.0% | <0.0001 |
| Men | 49.3% | 45.6% | <0.0001 |
| Chronic obstructive pulmonary disease | 30.0% | 25.3% | <0.0001 |
| Heart failure | 39.4% | 27.7% | <0.0001 |
| Hypertension | 58.8% | 57.9% | 0.196 |
| Diabetes mellitus | 25.9% | 23.1% | <0.0001 |
| Coronary artery disease | 56.6% | 43.8% | <0.0001 |
| Stroke (including transient ischemic attack) | 4.6% | 7.3% | <0.0001 |
| Hypothyroidism | 18.1% | 16.0% | <0.0001 |
| Chronic kidney disease | 21.3% | 15.2% | <0.0001 |
| Liver disease | 2.5% | 3.1% | 0.009 |
| Median CHADS2 score | 2.0 | 2.0 | 0.047 |
| Concurrent use of nonamiodarone rhythm-control medications | 0.5% | 10.6% | <0.0001 |
| Concurrent use of rate-control medications | 52.5% | 57.3% | <0.0001 |
| Concurrent use of strong cytochrome P450 3A4 inhibitors | 0.6% | 1.0% | 0.005 |
| Concurrent use of corticosteroids | 0.5% | 0.6% | 0.489 |
| Concurrent use of medications associated with pulmonary fibrosis | 0.7% | 1.0% | 0.031 |
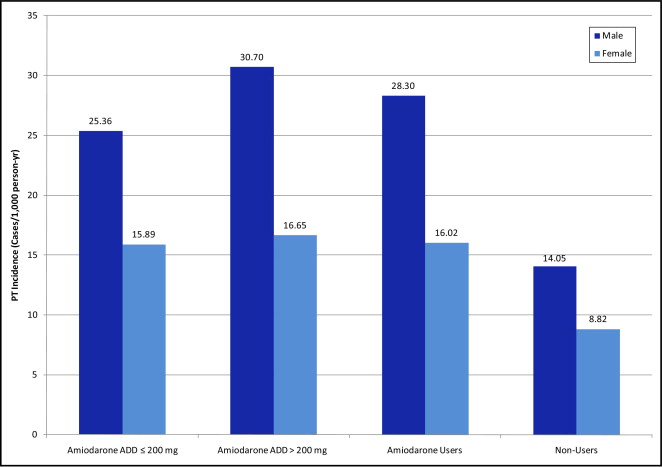
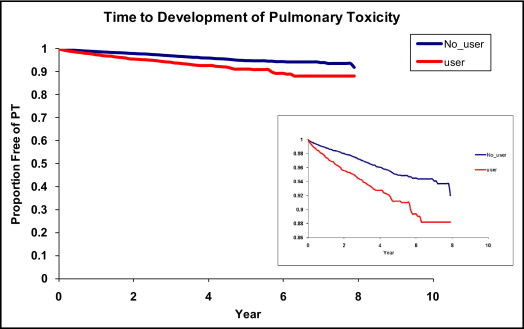
Crude incidences of PT were 3.87% (250 of 6,460) for users and 1.33% (676 of 50,993) for nonusers. The most common PT diagnosis was ICD-9 code 515.9 (postinflammatory pulmonary fibrosis; Table 2 ). A higher age-standardized incidence of PT was found in users than nonusers and in men than women ( Figure 1 ). Men and women users had incidences of 28.30 and 16.02 cases per 1,000 person-years, respectively, whereas men and women nonusers had estimates of 14.05 and 8.82 cases per 1,000 person-years, respectively. Age-standardized incidence of PT had a slight dose response, with an increase in incidence with doses ≥200 mg/day ( Figure 1 ). Incidences in users with and without COPD were 35.63 and 15.36 cases per 1,000 person-years, respectively, whereas in nonusers with and without COPD incidences were 21.80 and 7.59 cases per 1,000 person-years, respectively. PT events continued to cumulate over the follow-up period ( Figure 2 ). The crude mortality rate for those who developed PT was 75.7%.
| Postinflammatory pulmonary fibrosis | 368 (39%) |
| Other pulmonary insufficiency | 258 (27%) |
| Pulmonary insufficiency after trauma and surgery | 121 (13%) |
| Other interstitial pulmonary disorders | 82 (9%) |
| Other specified alveolar and parietoalveolar pneumonopathies | 44 (5%) |
| Idiopathic fibrosing alveolitis | 29 (3%) |
| Acute respiratory distress syndrome | 23 (2%) |
| Other (each <1% in frequency) | 23 (2%) |
| Total | 948 (100%) |
⁎ Some patients had >1 diagnosis type coded; therefore, coding diagnosis events = 948, whereas actual patient events = 926.
Amiodarone was associated with a significantly increased risk of PT when adjusting for potential confounders at doses ≤200 mg/day (hazard ratio 1.62, 95% confidence interval 1.35 to 1.96) and >200 mg/day (hazard ratio 1.46, 1.22 to 1.75). A significantly increased risk of PT was also associated with male gender, increased age, COPD, and CKD. Patients with COPD had the highest risk of developing PT (2.53, 2.21 to 2.89). Although patients who were taking strong cytochrome P450 3A4 inhibitors had a trend toward increased risk of PT, it was not significant (hazard ratio 1.53, 0.96 to 2.44), whereas subjects who were taking concurrent rate-control medications had a significantly lower risk of PT (hazard ratio 0.85, 0.75 to 0.97; Table 3 ). Sensitivity analyses including dose–gender and dose–age interaction terms found no evidence of interaction. Sensitivity analyses that defined use of amiodarone within 30 days instead of 7 days of index AF diagnosis revealed consistent estimates for risk of amiodarone PT (hazard ratio 1.84, 1.58 to 2.14; 1.79, 1.56 to 2.07, respectively).



