AMR101 is an omega-3 fatty acid agent containing ≥96% eicosapentaenoic acid ethyl ester and no docosahexaenoic acid. Previous smaller studies suggested that highly purified eicosapentaenoic acid lowered triglyceride (TG) levels without increasing low-density lipoprotein (LDL) cholesterol levels. TG-lowering therapies such as fibrates, and fish oils containing both eicosapentaenoic acid and docosahexaenoic acid, can substantially increase LDL cholesterol levels when administered to patients with very high TG levels (≥500 mg/dl). The present double-blind study randomized 229 diet-stable patients with fasting TG ≥500 mg/dl and ≤2,000 mg/dl (with or without background statin therapy) to AMR101 4 g/day, AMR101 2 g/day, or placebo. The primary end point was the placebo-corrected median percentage of change in TG from baseline to week 12. The baseline TG level was 680, 657, and 703 mg/dl for AMR101 4 g/day, AMR101 2 g/day, and placebo. AMR101 4 g/day reduced the placebo-corrected TG levels by 33.1% (n = 76, p <0.0001) and AMR101 2 g/day by 19.7% (n = 73, p = 0.0051). For a baseline TG level >750 mg/dl, AMR101 4 g/day reduced the placebo-corrected TG levels by 45.4% (n = 28, p = 0.0001) and AMR101 2 g/day by 32.9% (n = 28, p = 0.0016). AMR101 did not significantly increase the placebo-corrected median LDL cholesterol levels at 4 g/day (−2.3%) or 2 g/day (+5.2%; both p = NS). AMR101 significantly reduced non–high-density lipoprotein cholesterol, apolipoprotein B, lipoprotein-associated phospholipase A 2 , very low-density lipoprotein cholesterol, and total cholesterol. AMR101 was generally well tolerated, with a safety profile similar to that of the placebo. In conclusion, the present randomized, double-blind trial of patients with very high TG levels demonstrated that AMR101 significantly reduced the TG levels and improved other lipid parameters without significantly increasing the LDL cholesterol levels.
Triglyceride (TG)-lowering therapies such as fibrates, and fish oil agents containing a mixture of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), often substantially increase the low-density lipoprotein (LDL) cholesterol levels in hypertriglyceridemic patients, especially patients with very high TG levels. The treatment guidelines suggest LDL cholesterol is the primary lipid treatment target to reduce atherosclerotic coronary heart disease risk. Thus, an increase in LDL cholesterol levels when treating hypertriglyceridemic patients could complicate achievement of the LDL cholesterol treatment goals. Smaller trials of patients with normal to moderately elevated TG levels suggested that purified EPA might reduce TG levels without increasing the LDL cholesterol levels. AMR101 contains ≥96% of the omega-3 EPA ethyl ester, with no DHA. This 12-week study investigated the efficacy and safety of AMR101 in reducing TG levels and other lipid parameters in patients with very high TG levels (≥500 mg/dl).
Methods
The Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension (MARINE) was a Phase III study conducted in the United States, South Africa, India, Russia, Ukraine, Finland, Germany, Italy, and The Netherlands. The study was conducted under guidelines set forth by Good Clinical Practice, the Declaration of Helsinki, and all local and/or national regulations and directives. The protocol was approved by the appropriate institutional review boards, and all patients provided written informed consent before enrollment. The first patient was screened on December 14, 2009, and the last patient visit for the 12-week double-blind phase was October 19, 2010. The clinical trial registration number was NCT01047683 (available at: http://www.clinicaltrials.gov/ct2/show/NCT01047683? ).
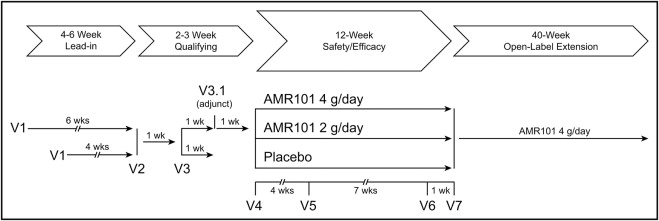
Figure 1 describes the study design. At the screening visit (visit 1), patients entered a diet, lifestyle, and medication stabilization period, wherein they were provided information regarding the National Cholesterol Education Program Therapeutic Lifestyle Changes Diet and were instructed to maintain the diet for the duration of the study. The duration of this stabilization period was 4 weeks for patients who were not on lipid-altering therapy or were on a stable dose of statins (with or without ezetimibe) and 6 weeks for patients who were required to discontinue prohibited lipid-altering therapy such as fibrates, niacin, and omega-3 fish oil. Patients were eligible to enter the 12-week double-blind period if, after the stabilization period, the average of the fasting TG levels at 2 visits separated by 1 week was ≥500 mg/dl and ≤2,000 mg/dl. If the average fasting TG was outside this range, an additional optional TG measurement was permitted 1 week later, and eligibility was based on the average fasting TG levels from the last 2 visits. Subjects were randomized 1 week later (visit 4) to 1 of 3 blinded treatment groups: AMR101 4 g/day (2 one-g capsules taken orally twice daily), AMR101 2 g/day (1 one-g capsule and 1 placebo capsule taken orally twice daily), or placebo (2 capsules administered orally twice daily, each capsule containing light liquid paraffin). All capsules contained 0.2% all-rac-α-tocopherol as an antioxidant.
The primary study end point was the placebo-corrected median percentage of change in TG from baseline to study end (week 12) in the 2 active treatment groups compared to placebo. The baseline TG value was calculated as the average of the TG levels at the randomization visit (visit 4) and at the visit 1 week earlier. The TG value at study end was calculated as the average of the TG levels at the week 11 and week 12 visits. Additional efficacy end points included percent change from randomization baseline (1 value) to week 12 (1 value) for the secondary variables of very low density lipoprotein cholesterol, apolipoprotein B, and lipoprotein-associated phospholipase A 2 , and the exploratory variables of total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, very low density lipoprotein–TG, and non-HDL cholesterol.
The study included men or women >18 years of age willing to maintain a stable diet and not alter their normal physical activity level throughout the study. Women who were pregnant, planning to become pregnant, or breastfeeding were excluded. Women of childbearing potential were required to use accepted birth control methods throughout the study. Other than the TG criteria of ≥500 mg/dl and ≤2,000 mg/dl, no other lipid criteria were required for eligibility. Other exclusion criteria included a history of pancreatitis; body mass index >45 kg/m 2 ; weight change >3 kg during the lead-in period; hemoglobin A1c >9.5% (patients with diabetes mellitus were required to be receiving stable therapy); history of stroke, myocardial infarction, life-threatening arrhythmia, or coronary vascularization within 6 months before screening; thyroid-stimulating hormone >1.5 × upper limit of normal; clinical evidence of hypothyroidism or thyroid hormonal therapy that had not been stable for ≥6 weeks before screening; alanine aminotransferase or aspartate aminotransferase >3 × upper limit of normal; an unexplained creatine kinase concentration >3 × upper limit of normal or creatine kinase elevation owing to known muscle disease (e.g., polymyositis, mitochondrial dysfunction); blood donation of ≥1 pint (0.5 L) within 30 days before screening or plasma donation within 7 days before screening; the consumption of >2 alcoholic beverages per day after screening; a history of illicit drug use within 1 year before screening; a history of symptomatic gallstone disease unless treated with cholecystectomy; known nephrotic syndrome or >3 g/day proteinuria; and a history or evidence of major and clinically significant disease that could adversely affect the conduct of the study or patient safety. Prohibited drugs included those for weight loss (including over-the-counter or supplemental agents); human immunodeficiency virus protease inhibitors; cyclophosphamide; isotretinoin; routine or anticipated use of systemic corticosteroids (local, topical, inhalation, or nasal corticosteroids were permitted). Other medications, including antihypertensives, antidiabetes mellitus drug therapies, tamoxifen, estrogens, and progestins were permitted as long as the doses were stable ≥4 weeks before screening and were continued unchanged throughout the study. Any lipid-altering drug therapy other than statins and ezetimibe, including niacin >200 mg/day, fibrates, fish oil, other products containing omega-3, or other herbal products or dietary supplements with potential lipid-altering effects were discontinued on entering the lead-in period. Patients deemed at high risk of coronary heart disease were able to continue with their stable dose of statins with or without ezetimibe. In other patients, lipid-altering therapies, including statins, were discontinued at the screening visit.
All laboratory measurements were performed by the central laboratory, Medpace Reference Laboratories (Cincinnati, Ohio, Navi Mumbai, India, and Leuven, Belgium), which maintained Part III certification by the Centers for Disease Control Lipid Standardization Program for lipids and accreditation from the College of American Pathologists. TGs and cholesterol were measured using enzymatic colorimetric tests (Olympus AU2700 or AU5400 Analyzer, Olympus, Center Valley, Pennsylvania) with calibration directly traceable to the Centers for Disease Control reference procedures. HDL was isolated by precipitating apolipoprotein B–containing lipoproteins and chylomicrons with dextran sulfate, and HDL cholesterol was measured in the supernatant. LDL cholesterol, very low density lipoprotein cholesterol, and very low density lipoprotein–TG were measured after preparative ultracentrifugation (β quantification). Serum apolipoprotein B was measured using rate immune-nephelometry (Dade Behring BNII nephelometer, Siemens Healthcare Diagnostics, Deerfield, Illinois). Non-HDL cholesterol was calculated by subtracting HDL cholesterol from total cholesterol. Berkeley Heart Laboratory (Burlingame, California) measured lipoprotein-associated phospholipase A 2 concentration using the PLAC sandwich enzyme-linked immunosorbent assay (diaDexus, South San Francisco, California).
A standard deviation of 45% in the TG measurements and a significance level of p <0.01 required a sample size of 69 completed patients per treatment group to provide ≥90% power to detect a difference of 30% between AMR101 and placebo in the percentage of change from baseline in the fasting TG levels. Assuming a 15% dropout rate from randomization to completion of the double-blind treatment period, a total of 240 randomized patients was planned (80 patients per treatment group). The randomized population was defined as all patients who signed the informed consent form and were assigned a randomization number at visit 4. The intent-to-treat population consisted of all randomized patients who had a baseline efficacy measurement, received ≥1 dose of study drug, and had ≥1 postrandomization efficacy measurement. The per protocol population consisted of all intent-to-treat patients who completed the 12-week, double-blind treatment period without any major protocol deviations. The safety population was defined as all randomized patients who had received ≥1 dose of study drug. The efficacy evaluations were performed on the intent-to-treat population. The primary efficacy analysis was performed using a Wilcoxon rank sum test with the Hodges-Lehmann median and interquartile range. To control the family-wise error rate when performing multiple pairwise tests between the 2 dose levels of AMR101 and placebo, a predefined step-down testing procedure was followed with a fixed testing order. First, the percentage of change in the fasting TG from baseline to study end (week 12) was compared between AMR101 4 g/day and placebo. If this first comparison showed a statistically significantly greater TG-reducing effect compared to placebo at the prespecified significance level of p = 0.01, AMR101 2 g/day was also tested versus placebo as a primary end point. For the secondary and exploratory end points, the comparison of each variable from baseline to week 12 was made for each AMR101 treatment group with the placebo treatment group, using a significance level of p = 0.05.
The safety evaluation was done using reported adverse events, clinical laboratory assessments, 12-lead electrocardiographic findings, physical examination findings, weight, body mass index, and vital signs (heart rate and blood pressure). Adverse events were recorded by the investigator (who was unaware of the treatment regimen) as related or not related to the study drug. Treatment-emergent adverse events were defined as those adverse events that had newly occurred or had worsened in severity during the double-blind treatment period. The trial was designed and sponsored by Amarin Pharma Inc. (Mystic, Connecticut). Medical monitoring, data management, and statistical analyses were performed by Medpace Inc. (Cincinnati, Ohio). Dr. Bays (principal investigator) wrote the first draft of the present report, and subsequent drafts were revised and edited by all the investigators, who vouch for the accuracy and completeness of the data.
Results
The baseline demographics of the patients are listed in Table 1 and were comparable across the treatment groups. In general, most patients were overweight (mean body mass index 30.8 kg/m 2 ), white (88%), and male (76%), with a mean age of 52.9 years. Of the randomized patients, 24.9% received background statin therapy, 27.5% had diabetes mellitus, and 55.0% were at a high risk of coronary heart disease according to the patients’ medical histories. For the randomized population, the median TG level was 679.5 mg/dl, with 39.3% of these patients having a baseline TG level >750 mg/dl. The median baseline LDL cholesterol level was 86.0 mg/dl in the intent-to-treat population.
| Characteristic | AMR101 4 g/day (n = 77) | AMR101 2 g/day (n = 76) | Placebo (n = 76) | Total (n = 229) |
|---|---|---|---|---|
| Age, mean (SD) (years) | 51.9 ± 10.27 | 53.4 ± 9.34 | 53.4 ± 8.34 | 52.9 ± 9.34 |
| Age ≤65 years | 70 (91%) | 70 (92%) | 71 (93%) | 211 (92%) |
| Men | 59 (77%) | 58 (76%) | 58 (76%) | 175 (76%) |
| White | 67 (87%) | 67 (88%) | 68 (90%) | 202 (88%) |
| Weight, mean (SD) (kg) | 93.2 ± 18.27 | 92.1 ± 15.57 | 93.0 ± 16.92 | 92.8 ± 16.89 |
| Body mass index, mean (SD) (kg/m 2 ) | 30.4 ± 4.29 | 30.8 ± 4.24 | 31.0 ± 4.25 | 30.8 ± 4.25 |
| Statin use | 20 (26%) | 19 (25%) | 18 (24%) | 57 (25%) |
| Baseline triglycerides >750 mg/dl | 29 (38%) | 29 (38%) | 32 (42%) | 90 (39%) |
| Diabetes mellitus | 22 (29%) | 20 (26%) | 21 (28%) | 63 (28%) |
Figure 2 details the disposition of the 610 patients initially screened and the 229 subjects ultimately randomized. Of the 333 patients who were not included according to the inclusion/exclusion criteria, most (n = 250) had TG levels out of the specified range. More than 90% of each group completed the 12-week study.
The results of the primary end point are shown in Figure 3 and listed in Table 2 . In the intent-to-treat population, AMR101 4 g/day reduced placebo-corrected median TG levels by 33.1% (p <0.0001); AMR101 2 g/day reduced placebo-corrected median TG levels by 19.7% (p = 0.0051). The results for the per-protocol population were consistent with the results for the intent-to-treat population. Table 2 also includes the median percentage of reductions in TG levels in subgroups of the intent-to-treat population. In statin-treated patients, AMR101 4 g/day reduced the placebo-corrected median TG levels by 65% (p = 0.0001) and AMR101 2 g/day reduced the placebo-corrected median TG levels by 40.7% (p = 0.0276). Among the patients with a baseline TG level >750 mg/dl, AMR101 4 g/day reduced the placebo-corrected median TG levels by 45.4% (p = 0.0001). AMR101 2 g/day reduced the placebo-corrected median TG levels in this subgroup by 32.9% (p = 0.0016).
| Variable | AMR101 4 g/day (n = 76) | AMR101 2 g/day (n=73) | Placebo (n = 75) | Placebo-Corrected Median TG Change from Baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline TG (mg/dl) | End-of-Treatment TG (mg/dl) | Change from Baseline (%) | Baseline TG (mg/dl) | End-of-Treatment TG (mg/dl) | Change from baseline (%) | Baseline TG (mg/dl) | End-of-Treatment TG (mg/dl) | Change from baseline (%) | AMR101 4 g/day vs Placebo (%, p Value) | AMR101 2 g/day vs Placebo (%, p Value) | |
| Intent-to-treat population (n = 76, 73, 75) | 679.5 (265.3) | 502.0 (302.0) | −26.6 (41.2) | 656.5 (303.5) | 605.5 (415.00) | −7.0 (48.7) | 703.0 (426.5) | 745.5 (886.5) | 9.7 (61.6) | −33.1, <0.0001 | −19.7, 0.0051 |
| Baseline triglyceride >500 mg/dl (n = 66, 61, 64) | 710.3 (234.5) | 565.3 (389.0) | −27.4 (40.8) | 706.0 (295.5) | 628.5 (408.5) | −10.4 (44.0) | 749.5 (455.5) | 902.8 (899.3) | 10.1 (57.4) | −35.7, <0.0001 | −24.9, 0.0007 |
| Baseline triglyceride ≤750 mg/dl (n = 48, 45, 43) | 613.8 (155.5) | 454.5 (255.0) | −26.6 (32.0) | 568.0 (125.0) | 512.0 (219.0) | −7.0 (46.5) | 564.5 (144.5) | 592.5 (327.0) | 2.2 (58.7) | −25.1, 0.0006 | −9.1, 0.282 |
| Baseline triglyceride >750 mg/dl (n = 28, 28, 32) | 902.0 (477.3) | 680.5 (720.8) | −26.6 (48.7) | 947.5 (230.0) | 864.8 (460.0) | −7.3 (48.8) | 1,052 (541.3) | 1,423 (828.3) | 19.0 (56.6) | −45.4, 0.0001 | −32.9, 0.0016 |
| Statin use at baseline (n = 19, 19, 18) | 650.0 (210.5) | 448.5 (249.5) | −29.5 (42.7) | 591.5 (490.5) | 651.0 (340.0) | 11.1 (47.7) | 713.3 (471.0) | 949.5 (1,001.0) | 32.2 (95.0) | −65.0, 0.0001 | −40.7, 0.0276 |
| No statin use at baseline (n = 57, 54, 57) | 679.5 (251.0) | 579.5 (389.0) | −26.4 (34.1) | 672.8 (263.0) | 595.8 (444.0) | −10.2 (45.6) | 650.0 (379.5) | 675.5 (783.5) | 6.4 (56.1) | −25.8 0.0002 | −16.4 0.0360 |
Neither AMR101 4 g/day nor 2 g/day significantly increased the LDL cholesterol levels in either the overall intent-to-treat population or the intent-to-treat population subgroup with a baseline TG level >750 mg/dl ( Table 3 ). Regarding other end points, AMR101 4 g/day significantly reduced non-HDL cholesterol by 17.7% (p <0.0001), lipoprotein-associated phospholipase A 2 by 13.6% (p = 0.0003), very low density lipoprotein–TG by 25.8% (p = 0.0023), and apolipoprotein B by 8.5% (p = 0.0019). AMR101 2 g/day significantly reduced non-HDL cholesterol by 8.1% (p = 0.0182). Both AMR101 4 and 2 g/day significantly reduced very low density lipoprotein cholesterol and total cholesterol, with no significant effect on HDL cholesterol.



