Aspirin has been shown to decrease cardiovascular (CV) events by ∼25%. Despite aspirin therapy 10% to 20% of patients with arterial vascular disease develop atherothrombotic events. A meta-analysis of antiplatelet therapy showed a progressive decrease in clinical efficacy of aspirin after 2 years. Whether this is due to a decreased sensitivity to aspirin during long-term therapy remains unclear. A prospective randomized clinical trial with serial monitoring over 5 years was conducted in 100 patients with documented coronary heart disease. We investigated whether long-term treatment with aspirin 50 and 100 mg affects platelet response similarly. Occurrence of CV events was documented. Platelet sensitivity to aspirin, prostacyclin, and adenosine diphosphate-, collagen-, and epinephrine-induced platelet aggregation were evaluated over time. In addition, β-thromboglobulin and inflammatory markers were measured. Four patients were lost to follow-up and 10 patients died. Eleven patients developed nonfatal CV events. In the 2 groups platelet response to aspirin and the referenced variables remained unchanged over 5 years. In patients who developed CV events, the last monitoring interval revealed no difference in platelet response to aspirin. However, patients with nonfatal and fatal CV events showed increased inflammatory markers versus patients without CV events independent of aspirin 50 or 100 mg intake. In conclusion, our study revealed no difference in antiplatelet response to aspirin 50 versus 100 mg or CV events over 5 years in patients with coronary heart disease.
Aspirin as an antiplatelet drug has been found to prevent vascular death by ∼15% and nonfatal vascular events by ∼30% as reported in a meta-analysis. However, risk of a recurrent vascular event remains relatively high, with estimates of 8% to 18%, particularly after 2 years of treatment. It is unclear whether aspirin dose is too low in these patients, patients are not compliant, have a decreased absorption of aspirin, have an underlying genetic disposition that results in ineffectiveness, or because of the multifactorial nature of atherothrombosis. Furthermore, Pulcinelli et al reported that inhibition of platelet aggregation by aspirin progressively decreases in long-term treated patients. Two previous studies have evaluated the effect of platelet aggregation in patients undergoing prolonged aspirin treatment; however, the results were conflicting. In this study we assessed platelet aggregation in response to several agonists in patients treated with aspirin 50 or 100 mg for 60 months in a prospective randomized clinical trial to clarify whether this response is caused by chance or whether prolonged treatment may decrease sensitivity to aspirin. In addition, we measured inflammatory markers and documented fatal and nonfatal cardiovascular (CV) events.
Methods
From 1999 through 2005, 100 patients with documented coronary heart disease (CHD) were randomized 1:1 to evaluate platelet response to aspirin (Thrombo ASS, Lannacher Heilmittel, Austria) 50 versus 100 mg/day. Angiographically assessed coronary stenosis (>50%) was documented in 82% of patients taking aspirin 50 mg and in 72% who took 100 mg. Evidence of myocardial ischemia at single-photon emission computed tomography was assessed in 18% and 24%, and angiography and single-photon emission computed tomography were performed in 58% and 48%, respectively. The study was conducted in agreement with the Declaration of Helsinki and approved by an ethics committee; informed consent was obtained from all subjects. Patients were excluded if they had been taking another antithrombotic or nonsteroidal anti-inflammatory drug within 30 days before platelet function assessment, or if they were on an oral anticoagulant medication, had a peripheral platelet count <125 × 10 9 /L, hematocrit value <25%, or serum creatinine level >1.5 mg/dl. Arterial hypertension, diabetes, and hyperlipidemia were defined according to guidelines. The 2 groups were comparable in baseline demographics and laboratory parameters without significant differences ( Table 1 ).
| Variable | Aspirin 50 mg/day (n = 50) | Aspirin 100 mg/day (n = 50) |
|---|---|---|
| Age (years), mean (range) | 56 (38–69) | 56 (37–67) |
| Men | 58% | 60% |
| Body mass index (kg/m 2 ) | 28.8 ± 2.1 | 29.1 ± 1.9 |
| Current smokers | 40% | 28% |
| Hypertension | 22% | 28% |
| Diabetes mellitus | 16% | 24% |
| β Blockers | 46% | 42% |
| Nitrates | 54% | 50% |
| Statins | 62% | 66% |
| Angiotensin-converting enzyme inhibitors | 36% | 38% |
| Thyroid hormones | 8% | 6% |
| Hypercholesterolemia | 38% | 34% |
| Combined hyperlipidemia | 20% | 22% |
| Total cholesterol (mg/dl) ⁎ | 211 ± 19 | 208 ± 14 |
| High-density lipoprotein cholesterol (mg/dl) ⁎ | 47 ± 5 | 48 ± 4 |
| Low-density lipoprotein cholesterol (mg/dl) ⁎ | 112 ± 12 | 108 ± 10 |
| Triglycerides (mg/dl) ⁎ | 262 ± 41 | 260 ± 36 |
| Total cholesterol (mg/dl) † | 214 ± 23 | 212 ± 19 |
| High-density lipoprotein cholesterol (mg/dl) † | 48 ± 5 | 46 ± 5 |
| Low-density lipoprotein cholesterol (mg/dl) † | 113 ± 16 | 110 ± 13 |
| Triglycerides (mg/dl) † | 265 ± 38 | 282 ± 44 |
| Hematocrit (%) | 41.8 ± 4.3 | 41.8 ± 3.8 |
| Platelet count (10 9 /L) | 215.4 ± 56.3 | 210.8 ± 60.2 |
| High-sensitivity C-reactive protein (mg/dl) | 0.8 ± 0.3 | 0.8 ± 0.4 |
| Erythrocyte sedimentation rate 1 (mm) | 13.1 ± 3.9 | 12.2 ± 4.0 |
| Erythrocyte sedimentation rate 2 (mm) | 26.5 ± 7.4 | 24.9 ± 5.9 |
| Vascular cell adhesion molecule-1 (ng/ml) | 619.5 ± 54.1 | 609.4 ± 60.1 |
| Fibrinogen (mg/dl) | 264.5 ± 38.2 | 250.2 ± 40.0 |
⁎ Cohort on statin (aspirin 50 mg/day n = 31, aspirin 100 mg/day n = 33).
Platelet sensitivity to aspirin, prostaglandin I 2 , and adenosine diphosphate-, collagen-, and epinephrine-induced platelet aggregation was assessed before the start of treatment, 2 weeks thereafter, and at 1 month and then 3, 6, 12, 18, 24, 36, 48, and 60 months. In addition, β-thromboglobulin, inflammatory markers such as high-sensitivity C-reactive protein (CRP), fibrinogen, erythrocyte sedimentation rate, and vascular cell adhesion molecule-1 were measured. Patient compliance was assessed at all follow-up intervals.
The primary end point was to determine if platelet response to low-dose aspirin (50 vs 100 mg/day) varied over time. Secondary end points were occurrence of fatal (CV death) and nonfatal CV events during 5-year follow-up and possible existence of correlation with inflammatory markers. Information about cause of death or clinical events was obtained from hospital charts.
Blood samples were collected by antecubital venepuncture without stasis before aspirin intake in the morning and after an overnight fast of >12 hours. Hematocrit and platelet count were determined by an automated cell counter (SF 3000, Sysmex Austria, Vienna, Austria). Total cholesterol and triglyceride concentrations were measured by an automated enzymatic method (Roche Diagnostics GmbH, Mannheim, Germany). High-density lipoprotein cholesterol and low-density lipoprotein cholesterol were determined enzymatically after separation by ultracentrifugation (Roche Diagnostics GmbH). Erythrocyte sedimentation rate was determined by the Westergren method and high-sensitivity CRP by an immunonephelometric assay (Dade Behring, Marburg, Germany). Intra-assay and interassay variability coefficients were 2.8% and 1.7%. Fibrinogen was determined according to the method of Clauss. Vascular cell adhesion molecule-1 was measured with a commercially available enzyme-linked immunosorbent assay (R&D Systems Europe, Ltd., Abingdon, United Kingdom). Intra-assay and interassay variability coefficients were 5.3% and 2.9%.
Citrated (3.8% citrate) blood was centrifuged (150 g , 5 minutes) to obtain platelet-rich plasma (PRP). Platelet count of PRP was adjusted to 250 × 10 3 /μl with platelet-poor plasma, which had been centrifuged (1,500 g , 15 minutes) from PRP. Induced aggregation in PRP was measured in a Born-type aggregometer (APACT 2/4 channel, LABiTec GmbH, Ahrensburg, Germany). Platelet aggregation response within 5 minutes of agonist stimulation was recorded and expressed as percent maximal platelet aggregation.
Platelet aggregation was induced with adenosine diphosphate (Böhringer Mannheim, Mannheim, Germany) 1 μmol/L in 600-μl PRP samples 1 minute after addition of 3 concentrations of aspirin (1, 10 and 100 mg/L) to determine equal effects at all sampling times and 1 minute after addition of buffer solution (pH 7.4) 100 μl containing 3 different concentrations of antiaggregator prostaglandin I 2 . The 50% inhibitory dose was expressed in milligrams of aspirin per milliliter of PRP and in nanograms of prostaglandin I 2 per milliliter of PRP. Intra-assay and interassay variability coefficients were 2.7% and 4.8%.
Silicon-coated glass vials were filled with PRP 600 μl and stirred with a Teflon-coated magnetic stirrer (800 rpm). Aggregation was induced with adenosine diphosphate 1 μmol/L, collagen 1 μg (Collagen Reagent Horm, Hormon-Chemie, Munich, Germany), and epinephrine 5 μmol/L (Sigma-Aldrich GmbH, Vienna, Austria). In addition, transmission of a PRP sample was monitored for 10 minutes to assess spontaneous aggregation. Intra-assay and interassay variability coefficients were 3.9% and 6.1% for adenosine diphosphate, 3.6% and 5.7% for collagen, and 2.9% and 6.0% for epinephrine. Concentration of β-thromboglobulin was determined using a commercially available radioimmunoassay kit (Amersham International, Buckinghamshire, United Kingdom). Intra-assay and interassay variability coefficients were 3.7% and 4.9%.
Native blood was allowed to clot in a glass vial at 37°C in a water bath for exactly 60 minutes. Serum was obtained by centrifugation (1,500 g , 10 minutes, 4°C). Serum samples were divided into aliquots, stored at <−70°C, and thromboxane (TX) B 2 was determined radioimmunologically within 2 weeks in nonextracted samples (GE Healthcare Life Sciences, Buckinghamshire, United Kingdom). The specific antibody was provided by Bernhard A. Peskar, MD. Free and antibody-bound ligands were separated by dextran-coated charcoal. At serum 195 ng/ml, intra-assay and interassay variability coefficients were 4.1% and 5.9%.
All data were analyzed using SAS 9.1.3 (SAS Institute, Cary, North Carolina). Data are presented as mean ± SD. Sample size calculation for a statistically significant p value <0.05 to achieve 80% power was performed for all dependent variables. Dichotomous variables are described as counts and percentages. Repeated measures analysis of variance and t tests were calculated with Bonferroni adjustment and post hoc tests. Chi-square tests for medications used the Yates correction. A p value <0.05 was considered statistically significant.
Results
Hematocrit, platelet count, and inflammatory markers within and between groups were comparable at baseline ( Table 1 ) and not significantly different after 5 years of follow-up.
Results of sensitivity and aggregation tests were distributed in quintiles. The higher the quintiles, the lower the response was to aspirin in the aggregation tests and the lower the sensitivity of platelets to aspirin and prostaglandin I 2 . Our data demonstrated high interindividual but low intraindividual variability (data not presented) in platelet function at follow-up.
Platelet sensitivity tests showed a comparable response irrespective of aspirin intakes 50 or 100 mg. Overall the monitoring period for 50% inhibitory dose revealed no significant differences between or within aspirin treatment with 50 and 100 mg ( Figure 1 ). Over the 5-year monitoring period, the quintile distribution remained stable without significant changes.
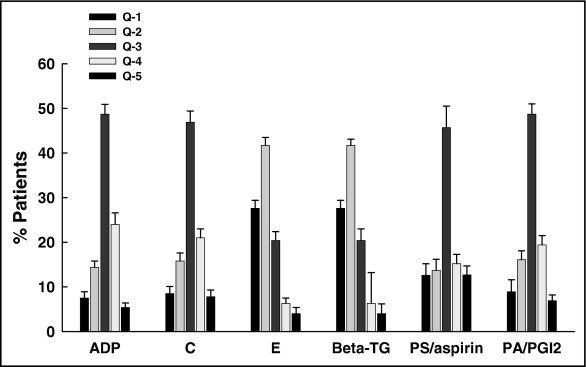
Percent maximal platelet aggregation remained stable over the serial assessments and at 5 years and was not significantly different. Similarly, tquintile distribution of the study population remained unchanged for each test over the 5-year follow-up ( Figures 1 and 2 ).
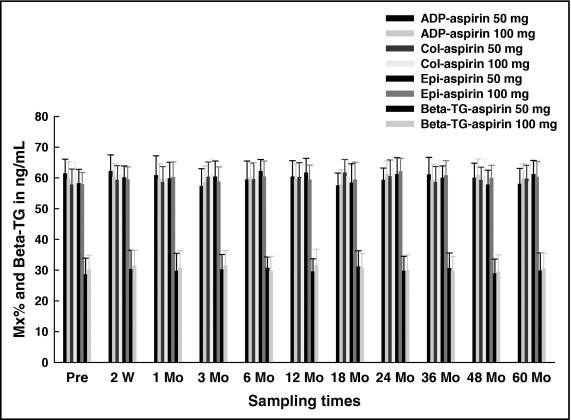
Beta-thromboglobulin revealed no significant differences during follow-up within and between 50- and 100-mg treatments ( Figure 2 ).
We found a high rate of compliance to aspirin therapy as determined by suppression of serial TXB 2 concentrations, face-to-face interview, and pill counting. At each assessment patients with serum TXB 2 >10 ng/ml were excluded from our statistical analysis. Therfore, we were able to eliminate noncompliance as a potential confounding variable ( Table 2 ).
| Follow-up (months) | Interview | Pill Count | TXB 2 >10 ng/ml | TXB 2 | |
|---|---|---|---|---|---|
| Aspirin 50 mg | Aspirin 100 mg | ||||
| 0.5 | 0 | 0 | 1 | 5.3 ± 3.8 | 4.9 ± 2.6 |
| 1 | 1 | 1 | 1 | 4.7 ± 2.9 | 5.4 ± 2.2 |
| 3 | 1 | 2 | 1 | 5.3 ± 3.3 | 5.0 ± 2.7 |
| 6 | 1 | 1 | 1 | 6.1 ± 2.9 | 5.1 ± 3.0 |
| 12 | 0 | 2 | 0 | 4.7 ± 4.1 | 4.9 ± 3.2 |
| 18 | 0 | 3 | 0 | 3.7 ± 3.2 | 4.0 ± 3.0 |
| 24 | 0 | 2 | 1 | 3.5 ± 2.1 | 3.7 ± 2.4 |
| 36 | 0 | 1 | 1 | 3.9 ± 2.8 | 3.8 ± 2.6 |
| 48 | 0 | 2 | 1 | 4.1 ± 2.4 | 4.0 ± 2.2 |
| 60 | 1 | 3 | 1 | 5.8 ± 1.9 | 5.1 ± 2.3 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


