The effectiveness of a pharmacoinvasive strategy consisting of fibrinolysis and transfer for percutaneous coronary intervention (PCI) compared to primary PCI (PPCI) in patients presenting to non–PCI-capable hospitals with ST-elevation myocardial infarction (STEMI) is not well defined. We analyzed data from the Mayo Clinic STEMI database of patients treated with a pharmacoinvasive strategy (favored in those presenting early after symptom onset) or PPCI in a regional STEMI network from 2004 to 2012. A total of 364 and 1,337 patients were included in the pharmacoinvasive and PPCI groups, respectively. Patients in the PPCI group were older and more frequently had cardiogenic shock at the time of presentation (12.1% vs 7.7%, p = 0.018). Death from any cause occurred in 58 (16%) and 314 (23%) patients in the pharmacoinvasive and PPCI groups, respectively (median follow-up 3.9 and 4.4 years, respectively). In multivariate analyses adjusting for age, gender, and other variables for which the 2 groups differed at baseline, there was no significant difference between the 2 strategies for 30-day (hazard ratio 0.66, 95% confidence interval 0.36 to 1.21) or overall mortality (hazard ratio 0.84, 95% confidence interval 0.63 to 1.12). Shorter door-to-balloon time was associated with increased effectiveness of PPCI (p for trend = 0.015), but there was no difference between the 2 strategies even when considering only the patients with door-to-balloon time in the lowest quartile. In conclusion, fibrinolysis followed by transfer for PCI represents a reasonable alternative when PPCI is not readily available especially in patients presenting early after symptom onset.
In patients with ST-elevation myocardial infarction (STEMI), the superiority of primary percutaneous coronary intervention (PPCI) over fibrinolysis has been shown by numerous studies, and PPCI remains the preferred strategy recommended by major guidelines when revascularization is possible soon after the first medical contact. However, when mechanical reperfusion with PPCI cannot be performed expeditiously, a pharmacoinvasive strategy of fibrinolysis followed by coronary angiography and PCI may be superior to delayed PPCI, especially in patients presenting very early after symptom onset. Fibrinolysis continues to be used as first-line therapy in >25% of patients with STEMI when geographic, weather, or traffic conditions do not permit prompt transfer to a PCI-capable hospital, and recent data suggest that fibrinolysis may be even underutilized. Much of the evidence for these guideline-recommended strategies and timelines is derived from randomized controlled trials (RCT), but the delivery of STEMI care outside clinical trials presents challenges and differences may exist between the results of RCTs and different practice settings. Describing the performance of different strategies as they are applied in clinical practice is important to identify barriers to optimal delivery of care and outcomes. In this retrospective analysis, we sought to compare the outcomes of patients treated with a pharmacoinvasive or a PPCI strategy in a large regional STEMI network around Mayo Clinic, Rochester, Minnesota.
Methods
The study population comprised patients diagnosed with STEMI at Mayo Clinic, Rochester, Minnesota, and patients referred to the institution from regional hospitals from May 17, 2004, to December 31, 2012. STEMI diagnosis was established based on electrocardiographic ST-segment elevation, cardiac biomarker elevation, and symptoms of ischemia. Patients with suspected initial STEMI diagnosis that was later refuted were excluded from this analysis. The Mayo Clinic Institutional Review Board approved this study. Patient consent for participation followed standard procedures in accordance with the institutional review board. In the event of patient refusal, the Ethics Committee was not approached for approval.
The Mayo Clinic STEMI protocol has been previously described in detail. Briefly, the protocol has been in use since May 2004, and its key components are rapid electrocardiogram acquisition and interpretation within 10 minutes of presentation, single-call activation of the catheterization laboratory without need for authorization by the accepting cardiologist, and ability of the laboratory to operate within 30 minutes from activation. Regional hospitals participated in the protocol if transfer to Mayo Clinic Hospital–Saint Marys Campus, Rochester, Minnesota, could be generally achieved in <90 minutes provided that there was no closer alternative facility with round-the-clock PCI capabilities. A total of 28 regional hospitals in southeastern Minnesota, Iowa, and Wisconsin participated in the original network, and another 14 joined the network at later stages ( Supplementary Table 1 ). The protocol recommended that patients presenting at regional hospitals <3 hours after symptom onset should receive full-dose intravenous fibrinolysis (reteplase or weight-based tenecteplase at the discretion of the regional hospital) followed by immediate transfer to Rochester, Minnesota, for rescue PCI (for failed fibrinolysis, defined as persistent chest discomfort or <70% resolution of ST elevation 60 to 90 minutes after fibrinolytic drug administration) or elective catheterization within 24 hours. Although transfer after fibrinolysis occurred with an intention for catheterization, patients did not undergo immediate catheterization unless clinically indicated. To minimize the impact of symptom duration recall biases and to improve the discrimination between “early” and “late” presenters, on February 20, 2010, the protocol was amended with a recommendation to treat with fibrinolysis if symptom onset was within 2 hours rather than 3 hours. Patients with symptom duration >3 hours (>2 hours after February 20, 2010), clinical suspicion for alternative diagnosis (such as pericarditis) or contraindications to fibrinolytics were transferred for immediate angiography with a view toward PPCI if deemed appropriate. Primary PCI was also favored in patients with cardiogenic shock at presentation (defined as persistent systolic blood pressure ≤85 mm Hg that was unresponsive to fluid administration and required vasopressors or mechanical circulatory support), but this was not an absolute contraindication for fibrinolysis. Despite these protocol-based recommendations, an individually tailored approach was allowed, and deviations from the protocol were left to the discretion of the referring and accepting teams based on patient- and non–patient-related factors. For example, PPCI could be used in regional patients presenting within 3 hours after symptom onset (<2 hours after February 20, 2010) despite the absence of absolute contraindications to fibrinolysis. Unless contraindicated, all patients received aspirin 325 mg and unfractionated heparin (loading dose of 60 U/kg with maximum 4,000 U, and continuous infusion at 12 U/kg/h with maximum 1,000 U/h), whereas clopidogrel 75 mg was given upfront only to patients in the pharmacoinvasive group at the time of fibrinolytic therapy administration. This clopidogrel dose (instead of 300-mg load) was chosen to minimize bleeding risk, especially in patients >75 years and is supported by previous randomized trial data. Use of glycoprotein IIb/IIIa inhibitors and other adjunctive medications was left to the discretion of the treating teams. When used, eptifibatide was chosen instead of abciximab because of easier storage and administration during transfer. Eptifibatide was administered with a loading dose of 180 μg/kg twice (10 minutes apart) and continuous infusion at 2 μg/kg/min (glomerular filtration rate ≥50 ml/min) or 1 μg/kg/min (glomerular filtration rate <50 ml/min). Door-to-balloon (DTB) time was defined as the time from the first hospital arrival to the time of the first balloon inflation or use of other therapeutic interventional device such as a stent or a thrombectomy catheter.
Continuous variables are summarized as mean (SD) or median (interquartile percentile [IQR]). Discrete variables are summarized as frequency (percentage). Group differences were tested using 2-sample Student t test for approximately symmetric continuous variables, the Mann–Whitney rank-sum test for skewed continuous or discrete ordinal variables, and Pearson chi-square test for nominal variables. All-cause mortality was the main end point of interest. Kaplan–Meier curves of all-cause mortality at 30-day and long-term follow-up are presented for the pharmacoinvasive and PPCI groups. Differences between the 2 groups were tested with the log-rank test. Cox proportional hazards models were used to estimate the effect of the pharmacoinvasive strategy versus PPCI on short- and long-term mortality. The proportional hazards assumption for the pharmacoinvasive effect was assessed using plots of the scaled Schoenfeld residuals. The potential for a differential effect after 30 days was modeled using time-dependent covariates. Adjusted analysis was performed including age, gender, and other variables significantly different at the 0.10 level between the 2 groups as covariates. Heart rate and blood pressure variables were modeled as 3 degree of freedom splines for covariate adjustment. Long-term follow-up was obtained from the Mayo Clinic Registration records, which combine information from patient visits and periodic patient correspondence. Analyses were conducted using SAS version 9.4 (SAS Inc., Cary, North Carolina). All hypothesis tests were 2 tailed with a 0.05 significance level.
Results
There were 1,913 confirmed STEMI diagnoses in 1,862 unique patients, of whom 161 refused use of their records for research. In the remaining patients, only the first STEMI event was kept for analysis. Thus, a total of 1,701 patients were included in this analysis (364 [21%] treated with the pharmacoinvasive strategy and 1,337 [79%] treated with PPCI). In the pharmacoinvasive group, after fibrinolysis, 295 (81%) patients underwent PCI (rescue n = 153 [42%], elective n = 142 [39%]), whereas 19 (5%) patients had coronary artery bypass graft surgery and 50 (14%) patients had no PCI at all (reasons not documented). Because an individually tailored strategy was allowed by the protocol, 223 (42%) of 536 regional patients presenting within 3 hours (or within 2 hours after February 20, 2010) underwent PPCI and 33 (13%) of 250 regional patients presenting with symptoms of longer duration received fibrinolysis. Characteristics of early regional presenters are summarized in Supplementary Table 2 . Symptoms were of unknown duration in 112 regional patients.
Patients in the PPCI group were older (mean 64.4 ± 14 vs 62.7 ± 13.2 years, p = 0.038) and more frequently carried a diagnosis of congestive heart failure and peripheral vascular disease ( Table 1 ). The Thrombolysis in Myocardial Infarction risk index was similar in the 2 groups (p = 0.50), but cardiogenic shock at the time of presentation was more common in the PPCI group (12.1% vs 7.7%, p = 0.018). Left ventricular ejection fraction at presentation (as measured by transthoracic echocardiography or left ventriculography) was not significantly different. Electrocardiogram-derived STEMI location was similar between the 2 groups, with inferior location being the most common. Information on adjunctive medications used at the time of presentation and on discharge from index hospitalization is provided in Table 2 . In the PPCI group, radial catheterization and thrombectomy were used in 10% and 31% of patients, respectively. Drug-eluting stents were used in 75% of PPCI cases.
| Variable | Pharmacoinvasive (n=364) | Primary PCI (n=1,337) | P-value |
|---|---|---|---|
| Age (years) | 62.7±13.2 | 64.4±14 | 0.038 |
| Men | 275 (75.5%) | 945 (70.7%) | 0.06 |
| BMI (kg/m 2 ) | 29.1±5.4, median 28.4 (IQR 25.1-32.5) | 29±6, median 28.1 (IQR 25-31.8) | 0.42 |
| Initial heart rate (beats per minute) | 74.5±19.5 | 76.6±19.1 | 0.07 |
| Initial SBP (mmHg) | 122±23 | 130.3±30.9 | <0.001 |
| Initial DBP (mmHg) | 74.5±15 | 77.4±19.2 | 0.008 |
| TIMI risk index | 25.3±13.8 | 26.7±16.6 | 0.50 |
| Cardiogenic shock | 28 (7.7%) | 162 (12.1%) | 0.018 |
| MI location | 0.57 | ||
| Anterior | 135 (37.1) | 526 (39.3%) | |
| Lateral | 36 (9.9%) | 141 (10.5%) | |
| Inferior | 191 (52.5%) | 657 (49.1%) | |
| CHF | 20 (5.5%) | 140 (10.5%) | 0.005 |
| Smoking history | 247 (67.9%) | 853 (63.8%) | 0.042 |
| Creatinine (mg/dL) | 1.1±0.5, median 1 (IQR 0.8-1.2) | 1.1±0.6, median 1 (IQR 0.8-1.2) | 0.14 |
| Hypertension | 230 (63.2%) | 838 (62.7%) | 0.99 |
| Hyperlipidemia | 227 (62.4%) | 837 (62.6%) | 0.75 |
| Diabetes mellitus | 58 (15.9%) | 243 (18.2%) | 0.31 |
| Prior MI | 68 (18.7%) | 223 (16.7%) | 0.36 |
| Prior PCI | 67 (18.4%) | 229 (17.1%) | 0.56 |
| PVD | 12 (3.3%) | 110 (8.2%) | 0.001 |
| Prior stroke | 19 (5.2%) | 101 (7.6%) | 0.12 |
| Medication | Pharmacoinvasive | Primary PCI | P-value |
|---|---|---|---|
| At presentation | n=364 | n=1,337 | |
| Aspirin | 358 (98.4%) | 1,286 (96.2%) | 0.13 |
| Beta-blocker | 271 (74.5%) | 750 (56.1%) | <0.001 |
| Glycoprotein IIb/IIIa inhibitor | 1 (0.3%) | 177 (33.3%) ∗ | <0.001 |
| At discharge | n=352 † | n=1,256 † | |
| Aspirin | 342 (97.2%) | 1,230 (97.9%) | 0.32 |
| Clopidogrel | 311 (88.4%) | 1,124 (89.5%) | 0.53 |
| Prasugrel | 3 (0.9%) | 15 (1.2%) | 0.82 |
| Statin | 339 (96.3%) | 1,198 (95.4%) | 0.21 |
| Beta-blocker | 251 (71.3%) | 847 (67.4%) | 0.16 |
| ACEi | 341 (96.9%) | 1,202 (95.7%) | 0.36 |
∗ Information available for 531 primary PCI patients.
Symptom-to-door time was significantly longer in the PPCI compared to the pharmacoinvasive group (median 114 vs 60 minutes; Table 3 ), which is largely protocol driven (fibrinolysis favored in those presenting early). In the PPCI group, median DTB time was 86 (IQR 58 to 126) minutes, and it was significantly shorter (median 65 minutes) for patients who presented directly to the referral center compared to regional patients (median 119 minutes). In the pharmacoinvasive group, median door-to-needle (DTN) time was 28 (IQR 20 to 37.5) minutes. There were no substantial trends in typical door-to-reperfusion metrics over time in the 3 groups. For regional patients, the median door-in door-out time was 65 (IQR 53 to 79) minutes for those receiving fibrinolysis and 65 (IQR 50 to 87) minutes for those transferred for PPCI.
| Time, median (IQR) (min) | Pharmacoinvasive (n=364) | Primary PCI (n=1,337) |
|---|---|---|
| Symptom-to-door | 60 (33-101) | 114 (54-263) |
| Door-to-balloon | NA | 86 (58-126) |
| Door-to-needle | 28 (20-37.5) | NA |
Twelve (3%; all inhospital) and 93 (7%; 81 inhospital) patients died from any cause at 30-day follow-up in the pharmacoinvasive and PPCI groups, respectively (total n = 105 [6%]; Figure 1 ). The pharmacoinvasive strategy was associated with reduced mortality compared to PPCI in univariate analysis (hazard ratio [HR] 0.48, 95% confidence interval [CI] 0.26 to 0.87) but not in multivariate analysis adjusting for age, gender, and other variables for which the 2 groups differed at baseline (initial blood pressure and heart rate, cardiogenic shock, smoking status, history of heart failure, and history of peripheral vascular disease; HR 0.66, 95% CI 0.36 to 1.21). Of note, there were 3 (1%) inhospital intracranial bleeding events in the pharmacoinvasive group (patients aged 74, 80, and 83 years) and no events in the PPCI group (p <0.001).
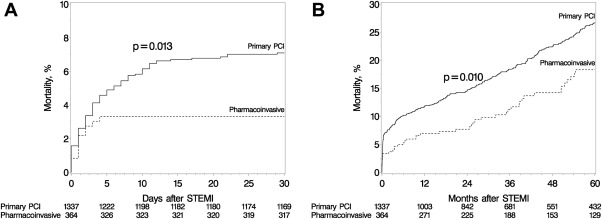
Median follow-up was 3.9 (IQR 1.3 to 6.8) and 4.4 (IQR 1.9 to 7.2) years in the pharmacoinvasive and PPCI groups, respectively. Among 1,596 early post-STEMI survivors, 1,485 (93%) were followed past 30 days. A total of 267 deaths from any cause occurred until the end of follow-up: 46 in the pharmacoinvasive and 221 in the PPCI group. At 1-year follow-up, the incidence of all-cause mortality was 7% in pharmacoinvasive patients and 12% in patients with PPCI. Among 30-day survivors, the annual mortality rates were 3.6%, 4.5%, and 4.3% for the pharmacoinvasive, PPCI, and overall patient populations, respectively. Among 30-day survivors, the 2 strategies had comparable effects on all-cause mortality in both univariate and multivariate analyses ( Table 4 ). The magnitude of the effect difference between the 2 groups tended to be larger for early compared to late mortality, but the difference was not statistically significant.
| Univariate HR (95% CI) | Multivariate HR (95% CI) ∗ | Multivariate HR (95% CI), early presenters ∗∗ | |
|---|---|---|---|
| Overall | 0.69 (0.52-0.92) | 0.84 (0.63-1.12) | 0.78 (0.57-1.08) |
| ≤30 days | 0.48 (0.26-0.87) † | 0.66 (0.36-1.21) ‡ | 0.74 (0.40-1.36) |
| >30 days | 0.79 (0.57-1.08) † | 0.92 (0.67-1.28) ‡ | 0.80 (0.55-1.16) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


