GENERAL CONSIDERATIONS
Arteriosclerosis, whether located within the coronary or noncoronary arteries, shares a similar pathophysiologic basis, starting with fatty streak and progressing to larger plaques with positive and negative remodeling leading to variable luminal compromise.
2,
3 Gradually, over many years plaque progression with noncompensatory remodeling leads to narrowing of the arterial lumen causing ischemia. In the peripheral arteries, occasionally, plaques may rupture leading to thrombosis and even embolization. Understanding this pathophysiologic process provides the basis for medical management of peripheral vascular disease (PVD). Given similar pathophysiologic mechanisms, coronary artery disease (CAD) and PVD share similar risk factors. These include family history of vascular disease, tobacco smoking, diabetes mellitus, hypertension, hyperlipidemia, advanced age, and inactivity.
4 Unfortunately, despite such similarity, PVD is frequently underdiagnosed and undertreated.
5,
6 More importantly, unlike CAD, risk factors such as hyperlipidemia in patients with PVD frequently are not treated as aggressively.
7 There is no doubt that PVD is the CAD risk equivalent and should be treated as vigorously with aggressive lipid lowering, blood pressure control, smoking cessation, diet, exercise, and antiplatelet therapy.
8
CAROTID ARTERIES
Approximately 800,000 individuals in the United States experience a stroke each year, with a 30-day mortality of 10%.
9,
10 Another 500,000 or more individuals suffer a transient ischemic attack (TIA) annually.
11 The risk is higher in women, with approximately 55,000 more women than men having a stroke each year.
12 Furthermore, blacks have an almost two-fold higher risk of first-stroke than whites.
13The majority of strokes (˜90%) are ischemic in nature; of these, only 15% to 20% are attributed to carotid artery stenosis.
14 Importantly, the severity of carotid artery stenosis is strongly predictive of stroke risk, with a 5-year risk of 7.8% in asymptomatic patients with less than 50% stenosis versus 18.5% in patients with 75% to 94% stenosis.
15 Furthermore, the presence of carotid artery disease is a significant marker of cardiovascular risk, highlighting the importance of risk factor modification and preventive strategies in this high-risk patient population.
16Carotid endarterectomy (CEA) has long been considered the gold standard for carotid artery intervention. In the last 50 years since CEA was initially reported, significant technical advancements have improved morbidity, mortality, cost, and patient comfort.
17 It was only after 40 years of widespread application of CEA, however, that definitive proof of its benefit was obtained. The landmark North American Symptomatic
Carotid Endarterectomy Trial (NASCET) and Asymptomatic Carotid Atherosclerosis Study (ACAS) investigations thus demonstrated that surgical endarterectomy, when performed for carotid bifurcation disease by experienced vascular surgeons on appropriately selected patients, effectively reduced the likelihood of ipsilateral stroke compared with standard medical therapy in both symptomatic and asymptomatic patients respectively.
18,
19 Indeed, a meta-analysis of all randomized clinical trials of CEA versus medical therapy show a 30-day risk of death and stroke of 3% for asymptomatic carotid disease and 6% for symptomatic patients.
20,
21Clinical trials have provided level I evidence for CEA for both symptomatic and asymptomatic patients with severe carotid disease.
18,
19 However, these studies have been limited by a number of factors. First, the patients selected for these trials were low to moderate risk. For example, presence of severe CAD or heart failure was an exclusion for many of these trials. Furthermore, medical therapy in that era was limited to aspirin and occasional lipid lowering agents. Therefore, contemporary data for CEA versus medical therapy are currently not available. Lastly, myocardial infarction (MI), a well-known complication of CEA, has not been included in the composite endpoints in any of these trials.
22Because of the limitations and the need for general anesthesia in many cases, a less invasive approach was developed. It was originally performed as balloon angioplasty alone, but later as a result of pioneering effort by Drs. Roubin, Iyer, Yadav, Vitek, and others, carotid stenting gained significant momentum.
23,
24,
25,
26,
27 In most institutions, percutaneous treatment of carotid arteries remained reserved for those circumstances wherein the surgical risk was very high.
24,
28 In 2001, Roubin published a 5-year single-center follow-up of 528 consecutive patients undergoing carotid stenting, with a 98% success rate, 1.6% mortality, and a combined endpoint of death and stroke of 7.4% at 30-day follow-up.
29 Of note, the rate of stroke decreased significantly over the 5-year study period, from 7.1% within the first year to 3.1% in the fifth year. This was felt to reflect improvements in technique, interventional devices, and pharmacotherapy. The seminal work of Roubin and colleagues demonstrated that the results of carotid stenting could be comparable with CEA and highlighted the need for randomized controlled studies to determine the optimal treatment strategy.
The first such randomized controlled trial was the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) trial.
30 The angioplasty procedure included stent insertion in only 26% of cases, the remainder were performed with balloon angioplasty alone. Distal protection was not available. Despite these limitations, the immediate and longterm results of angioplasty and endarterectomy were equivalent. Subsequently, smaller studies confirmed the benefits of carotid stenting, although the potential for distal embolization and consequent stroke remained a limiting factor (
Table 34.1). To address this concern, distal protection devices were developed to minimize or prevent distal embolization.
The first randomized trial comparing carotid stenting with emboli protection to CEA was the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial.
31 The SAPPHIRE study randomized 307 patients from 29 American centers to either carotid artery stenting (CAS) with distal protection or CEA. Entry criteria included asymptomatic carotid stenosis (>80% by ultrasound) or symptomatic stenosis (>50%), plus at least one feature placing the patient at higher risk for surgical endarterectomy. These features included age older than 80 years, the presence of congestive heart failure, severe chronic obstructive pulmonary disease (COPD), previous endarterectomy with restenosis, previous radiation
therapy or radical neck surgery, or lesions distal or proximal to the usual cervical location. Patients were screened by a team including a vascular surgeon, an interventionalist, and a neurologist. Consensus that the patient was a good candidate for both procedures was required before randomization; those rejected as surgical candidates underwent stenting and were included in a separate stent registry, whereas those rejected as candidates for intervention underwent surgery and were included in a surgical registry. At 30 days, the major adverse clinical events (MACE) were reduced by more than 50% with CAS as compared to surgery (5.8% for CAS, 12.6% for CEA,
P = 0.047). Interestingly, 408 patients in the SAPPHIRE study were deemed inappropriate for CEA and were therefore enrolled in the stent arm. Only seven patients were deemed inappropriate for carotid artery stenting.
31More recently, five randomized trials have evaluated the role of CEA versus CAS (
Table 34.2). Endarterectomy versus Stenting in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S), Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE), and International Carotid Stenting Study (ICSS) were conducted in Europe and enrolled patients with symptomatic carotid disease only.
32,
33,
34 These trials collectively failed to show equivalency between CAS and CEA. Indeed, CAS was associated with significantly higher 30-day stroke risk. Because of these data the Center for Medicare Services (CMS) has limited the use of CAS for asymptomatic high-risk individuals only.
35 However, despite being randomized, these trials have been widely criticized for including operators with minimal CAS experience and for their failure to use embolic protection devices.
36 In 2010, the long awaited National Institute of Health sponsored Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) was finally published.
37 Unlike the three European trials, CREST showed equivalent 30-day outcome between CEA and CAS for the composite endpoint of death, stroke, and MI. Furthermore, CEA was associated with higher periprocedural MI, and cranial nerve damage whereas CAS had higher incidence of minor stroke.
37 Collectively, the data from randomized clinical trials to date clearly demonstrate that a personalized approach to CAS and CEA would most likely result in the best outcome. Therefore, clinical and anatomic features including operator experience should guide the best revascularization strategy in patients with severe carotid disease (
Table 34.3).
Concomitant Carotid and Coronary Artery Disease
Around 7% to 10% of patients with severe CAD (left main trunk or three-vessel disease) have concomitant severe carotid disease.
38 While most experts agree that symptomatic carotid disease should be treated prior or in conjunction with coronary bypass graft (CABG) surgery, little consensus exists for asymptomatic carotid disease.
39,
40,
41 The risk of perioperative stroke in low-risk patients with asymptomatic severe (>80% stenosis) carotid disease undergoing CABG surgery is around 2% to 3%.
42 However, most published reports of combined CEA and CABG or staged CEA followed by CABG surgery reveal a 9% to 12% 30-day risk of death, stroke, or MI.
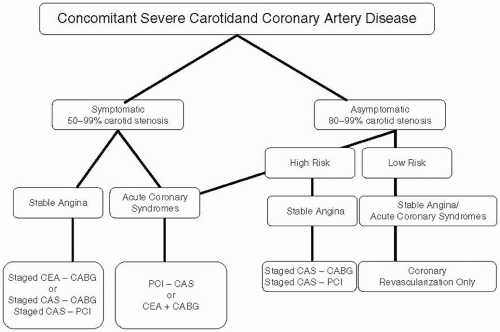
43 Based on the current available data we have recommended medical therapy for low-risk asymptomatic unilateral carotid disease in patients requiring CABG surgery (
Figure 34.1). For all other cases including symptomatic carotid disease stenting appears to be superior to the combined or staged CEA.
39
Treatment Considerations and Technique
Preprocedure Evaluation
A thorough history should be undertaken to determine symptom status. A full neurologic examination should then be performed by an individual certified in the NIH stroke scale.
44 A baseline carotid duplex, and ideally a computed tomography (CT) or magnetic resonance imaging (MRI) of the head, should be obtained. Once a severe stenosis is identified, clinical and anatomical features need to be considered and a decision regarding the best approach should be made in conjunction with the patient and family. We prefer premedication with aspirin and clopidogrel (at least 300 mg prior to intervention with sufficient time to achieve efficacy), and prehydration to minimize hypotension.
Angiographic Evaluation
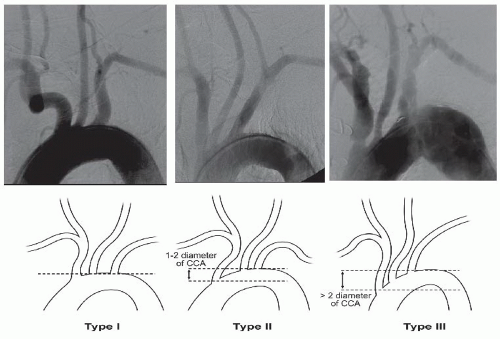
An arch aortography is performed under digital subtraction in the left anterior oblique (LAO) 30° to 40°. In this image the patient’s head should be turned toward the right and tilted upward. Arch aortography is important in identifying the aortic arch (whether it is type I, II, or III), which will impact endovascular approach and the selection of appropriate catheters in order to engage the carotid arteries (
Figure 34.2).
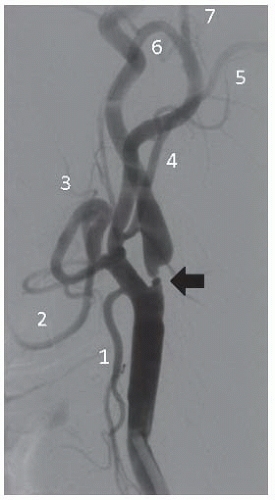
45 Additionally, the takeoff of carotid and vertebral arteries should be examined. Other factors such as degree of calcification, presence of ostial common carotid disease, and involvement of the external carotid artery may affect the procedural approach and success (
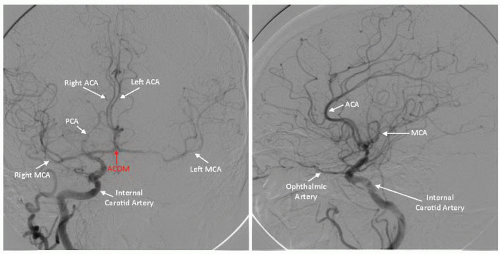
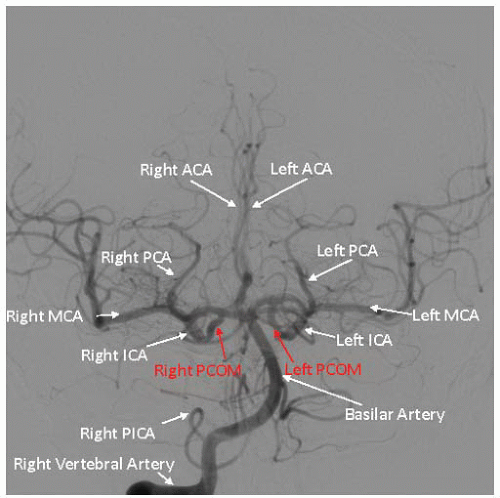
Figure 34.3). Subsequently, using appropriate diagnostic catheters such as 5F angled glide or JR4 for type I arch and Vitek, Simmons, or JB2 for more challenging anatomy, selective two-vessel (or four-vessel if indicated) cerebral angiography is performed. When performing selective angiography, the patient’s head should be secured to the table using carotid head gear. Images in the ipsilateral 30°-40° and lateral projections should be considered. Formal measurements must be made of the target lesion using NASCET criteria where distal reference is used beyond the carotid bulb. Intracranial cerebral angiography should be performed at baseline to rule out intracerebral arterial abnormalities and to establish the baseline arterial anatomy (
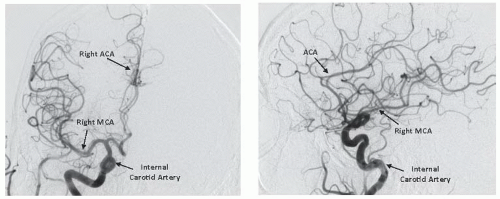
Figure 34.4). Of particular importance are the lesion morphology and the presence of collateral flow, patency of the
circle of Willis, and the dominance of the intracerebral arterial supply (
Figure 34.5).
46
Carotid Angioplasty and Stenting Procedure
A list of available equipment for carotid angioplasty and stenting is provided in
Tables 34.4 and
34.5. The femoral approach is typically used, although the radial approach in selected cases is possible and may be preferred. Almost all devices are 6F compatible, allowing the use of an 80-cm-long nonkinkable sheath (i.e., Cook Shuttle, Destination, Pinnacle, or ArrowFlex sheaths). Unlike a 90-cm sheath, the 80-cm sheaths are compatible with standard bailout equipments that are typically 100 cm long. We typically use a telescoping system for carotid artery intervention. A 5F long diagnostic catheter is placed inside a sheath once the sheath has already been advanced to the mid descending aorta. The carotid artery of interest is engaged using the diagnostic catheter. Subsequently, the glide wire is advanced into the external carotid artery. Occasionally the wire is left in distal common carotid artery under close watch not to accidently touch the lesion with sheath manipulation. The sheath is advanced up to the ostium of the innominate or left carotid artery. The 5F diagnostic catheter is then advanced to the distal common carotid artery holding the wire and sheath in place. The sheath is then advanced over the diagnostic catheter (or slipcath) in the common carotid artery. The process may require several iterations of advancing catheters and removing slack from the system to achieve final positioning. Once a desired location is reached, the glide wire and catheter are slowly removed to prevent air trapping. One should never cross the carotid lesion with a 0.035 inch wire or catheters.
Certain safety precautions are necessary for CAS procedures: 1) catheters should always be bled back to avoid any air or cholesterol embolization. 2) anticoagulation and anti-platelet therapy should commence prior to advancing any catheters into the carotid system. 3) less time in the carotid system is better. Although one should be cautious and deliberate in the performance of these procedures, the number of complications increases with additional intraarterial time. 4) catheter advancement should always be over a wire, and larger catheters should be transitioned in a stepwise, coaxial fashion over smaller catheters. 5) only atraumatic guidewires should be advanced into the internal carotid artery to minimize the risk of spasm or dissection. 6) predilatation is recommended to confirm the ability to adequately dilate the stenosis. 7) the use of self-expanding (SE) stents is preferred for the carotid bifurcation and other compressible sites.
47,
48 Balloon-expandable (BE) stents should be used only for aorto-ostial carotid lesions and distal (e.g., intracranial) internal carotid artery lesions. Eighth, when encountering resistance during advancement of balloons or stent delivery systems, removal and redilation with lower profile devices is appropriate. If an SE stent will not easily cross a predilated lesion, it should not be forced. Ninth, careful periprocedural hemodynamic monitoring is essential. Manipulation within the area of the carotid sinus can cause both acute and prolonged hypotension and bradycardia,
49 requiring fluid resuscitation, atropine or
α-adrenergic agents. Pacing is rarely needed but should be readily available.
50 Postprocedure hypertension must also be avoided, so as to minimize the chances of hyperperfusion syndrome, a potentially devastating entity that is occasionally seen following revascularization, particularly in elderly patients with previous near-occlusion and underperfused cerebral circulation. Tenth, postdilation should be performed to relatively low or nominal pressure. It is not necessary to eliminate completely the stenosis to achieve an excellent result.
51 Indeed, attempts to reduce the stenosis to 0% relative to the reference segment may lead to distal embolization, dissection, or resistant hypotension relating to carotid body stimulation or compression.
51
Stent Selection
If not all, the majority of stents used for carotid disease are nitinol SE stents; however, in rare cases we have used covered stents. Whether open or closed-cell design will have an important clinical significance is not well known. Newer hybrid stents, where the middle of stent is closed cell but ends are open are being developed.
52,
53 In general, the closed-cell design stents are more rigid (
Table 34.5).
Distal Protection
The combination of carotid stenting with distal protection has revolutionized carotid intervention (
Table 34.4). The first randomized trial demonstrating outstanding results of carotid stenting with distal protection was the SAPPHIRE
31 trial and this was quickly followed by several registries like ARCHeR
54 and SECuRITY.
55 In general, the available cerebral protection devices function either by filtering atheromatous debris out of the flowing blood distal to the lesion or by occluding antegrade flow proximal or distal to the lesion to allow removal of atheromatous debris. Each design has relative merits and potential disadvantages. The filtering devices allow for continuous visualization for precise stent placement and allow cerebral perfusion as antegrade blood flow is unobstructed. The current filter pore size ranges from 80 to 150 microns, raising the question of what diameter of atheromatous debris is required to cause neurologic sequelae. The occluding devices, by design, limit visualization and, in the absence of adequate collateral circulation, may result in prolonged cerebral ischemia. Retrograde embolization to the aortic arch may occur if aggressive injection is performed. However, occluding devices offer the theoretical advantage of protecting against a wider range of particulate sizes. The optimal protection device or combination of protection device and stent remains unclear and is a source of intense clinical investigation. However, early data indicate that proximal protection may have some advantage over distal embolic devices.
56
VERTEBRAL AND BASILAR ARTERIES
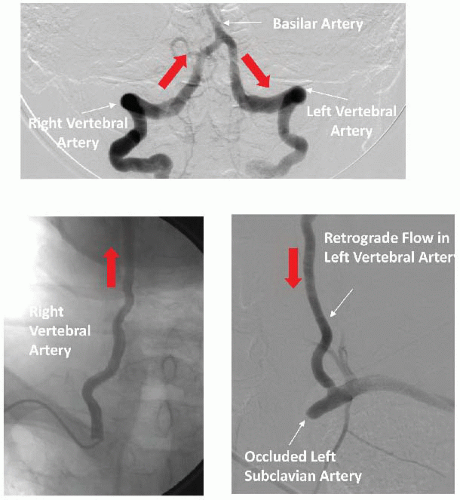
While vertebral artery stenosis is commonly encountered in patients undergoing arch or subclavian angiography, it rarely requires treatment. Intervention should be reserved for individuals with indisputable symptoms of vertebral basilar insufficiency (
Table 34.6). Occasionally, patients with occluded carotid arteries are dependent on vertebral blood flow. In these rare cases vertebral artery intervention may be necessary (
Figure 34.6). While any type of stroke could be devastating, posterior circulation or brain stem infarcts are extremely dangerous and could result in immediate death. Therefore, any decision regarding such interventions should
be made in consultation with neurology. Additionally, a thorough noninvasive assessment including MRI/MRA to evaluate the pattern of intracerebral blood flow and collateral circulation is paramount.
The basilar artery alone is responsible for perfusion to a number of critical areas in the brainstem. Accordingly, basilar artery angioplasty is reserved for those rare cases with acute occlusion or critical stenosis and symptoms related to posterior circulation. Intervention to this vessel may lead to occlusion of pontine branches or embolization to posterior circulation. Given the high risk involved in vertebral-basilar instrumentation, a multidisciplinary approach that includes a neurologist and a neuroradiologist is essential.
Treatment Considerations and Technique
Vertebral and basilar artery intervention should only be performed via a 0.014 inch system. The use of embolic protection devices is controversial and may lead to complications such as spasm or even plaque dislodgement.
61 In general, most experts agree that embolic protection is not required for all cases and should be considered on a case by case basis. A long 5F or 6F sheath is typically placed in the subclavian artery. Subsequently, the vertebral artery is wired and a small coronary balloon is advanced to the area of interest. Always lean toward undersizing in order to avoid dissection. Stenting is the best option for this location. Coronary stents are adequate in most situations and even drug-eluting stents can be used. Adequate coverage of the ostium is necessary to ensure long-term patency. Spasm is frequently encountered and may require nitroglycerine. Once a satisfactory result is obtained, a cerebral angiogram should be performed looking for abrupt vessel closure. A complete neurologic exam should be performed and patients should be monitored for at least 24 hours.