Introduction
Peripheral arterial disease (PAD) is usually defined as arterial disease which affects the arterial vasculature outside the heart. Aortic, renal and cerebral vascular diseases are addressed elsewhere in this text; therefore this chapter will focus on peripheral arterial disease of the lower extremities, distal to the aortic bifurcation.
The underlying pathologic abnormality of PAD of the lower extremities is obstruction of arterial flow, most commonly caused by atherosclerosis. However, other causes of arterial obstruction must also be entertained during diagnosis and include inflammatory diseases such as arteritis, entrapment syndromes, aneurysms and thrombosis in situ or thromboembolism. Atherosclerosis is a disease which primarily affects the intimal layer of elastic arteries, with the formation initially of a fatty streak, caused by adhesion moleculedependent infiltration of the intima of mononu-clear leukocytes, potentiated by chemoattractant chemokines. The leukocytes then become lipid laden, becoming macrophage foam cells, and the fatty streak is formed. Smooth muscle cells also infiltrate the plaque, with varying degrees of collagen formation. The resultant lipid-rich lesion, which is separated from the lumen of the artery only by a fibrous cap, can either rupture into the artery’ s lumen, triggering a thrombotic cascade and resulting in atherothrombosis and infarction, or become organized and ultimately even calcified, causing local obstruction to distal blood flow or stenosis.
PAD is an insidious condition, usually having a gradual onset and long latency before symptoms arise. It is associated with an increased risk of cardiovascular morbidity and mortality, and therefore evidence of PAD should direct health professionals to ensure that aggressive cardiovascular secondary prevention strategies are implemented.
Epidemiology and natural history
The prevalence of PAD depends on the age of the population surveyed and the method of diagnosis. The most commonly used method of PAD diagnosis is the Ankle Brachial Index (ABI). Data from several population-based studies indicate that PAD prevalence ranges between 3% and 10%, and increases to 15–20% among individuals over 70 years of age.1–3 The annual incidence of intermittent claudication is greater among men than women, and is reported as 4.1–12.9/1000 men and 3.3–8.2/1000 women aged 55 and over attending a primary care setting.4 The primary symptom of PAD of the lower extremities is limb claudication which is defined by a history of muscular leg pain on exercise that is relieved by a short rest. Claudication occurs when a stenosis in the artery restricts blood flow to the distal extremity to such an extent that when the person exercises and tissue oxygen demand increases, blood flow velocity cannot increase enough to cross the stenosis in adequate amounts to prevent tissue ischemia. Critical ischemia occurs when the arterial stenosis is so severe that blood flow is insufficient at rest, and the person has “rest pain”, which is initially resolved by placing the limb in a dependent position.
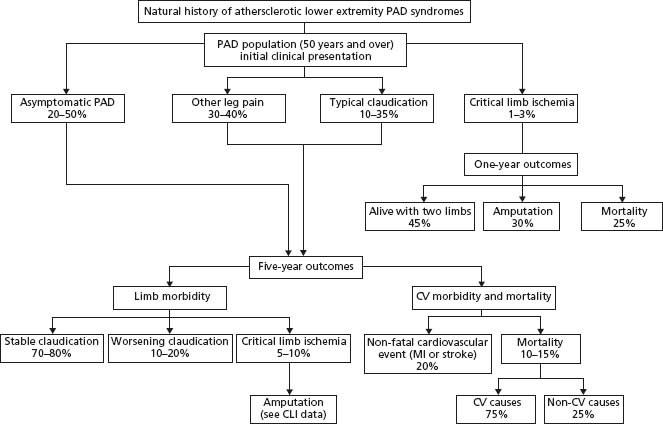
While the clinical interventions among patients with PAD traditionally focused on the impact of the arterial disease on the limbs, more recently the emphasis has shifted towards the risk of cardiovascular (CV) complications such as fatal and non-fatal myocardial infarction, and stroke faced by individuals with PAD. With respect to morbidity in the affected leg(s), only 1–3.3% of patients with intermittent claudication require amputation over a five-year period.5 However, some subgroups of PAD patients remain at higher risk of further limb pathology. Specifi-cally, 25% of patients with critical limb ischemia require amputation as the primary treatment5; patients who present with premature onset of PAD (i.e. at an age of ≤45 years) have an increased need for repeat limb intervention6 and those who continue to smoke after their PAD diagnosis have a worse prognosis for limb-related morbidity.7 A more ominous threat to patients with PAD is their poor CV prognosis, as approximately 40–60% of patients with PAD have concurrent coronary artery disease (CAD) and cere-brovascular disease. The annual major adverse cardiovascular event rate (myocardial infarction, ischemic stroke and vascular death) in PAD patients is 5–7%, with a five-year probability of a major non-fatal or fatal CV complication of 30–35%, and there is a 70% mortality rate after 15 years. A low ABI has also been shown to have a high specificity for future cardiovascular events, including stroke and myocardial infarction.8 Figure 65.1 shows the natural history of atherosclerotic lower limb PAD.
Figure 65.1 Natural history of atherosclerotic lower limb PAD over five years. PAD, peripheral arterial disease; CLI, critical limb ischemia; C V, cardiovascular; MI, myocardial infarction. (From Hirsch et al.18, with permission.)
Risk factors for PAD are similar to the major risk factors of atherosclerosis, although the relative impact of each risk factor varies somewhat. The major risk factors for PAD include male sex, tobacco exposure, advancing age, dysli-pidiemia, diabetes, and hypertension.
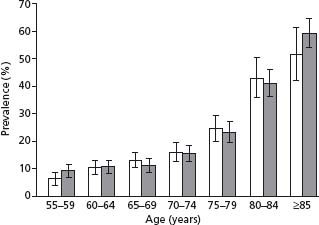
Male sex is reported as a PAD risk factor, as there are more men than women with PAD in younger age groups, which is likely due to the greater prevalence of smoking among men than women. However, because of the increased life span of women compared with men, and the increased incidence of PAD with increasing age, the overall incidence of PAD is actually similar in both sexes (Fig. 65.2). Tobacco exposure is the major risk factor for PAD, with the risk in current smokers being 2.3 times that of non-smokers.9 In one population-based study, the prevalence of current or former smoking in persons with PAD with an ABI of less than 0.9 was 85% in men and 47% in women,10 which results in a population attributable risk of tobacco exposure for PAD of 50–76%.9 Furthermore, the degree of tobacco exposure has a dose–response relationship with PAD risk, increasing with increasing pack-years of smoking. Other risk factors for the development of PAD include increasing age (see Fig. 65.1), dyslipidemia, diabetes (increase in PAD risk of 2–4-fold3), hypertension (increase in PAD risk of 2–4-fold11), impaired renal function (increase in PAD risk of threefold in women with a creatinine clearance of <30mL/min/1.73m212), and prior history of coronary artery disease (increase in PAD risk of 1.5-fold11). Dyslipidemia is a well-recognized risk factor, with an association between decreased ABI and increased total cholesterol and decreasing HDL cholesterol shown in the Cardiovascular Health Study.13 Similarly, a trend towards decreased ABI with higher triglyceride levels was noted, though this did not persist in multivariate analysis.13 Persons with diabetes mellitus are at particular risk of developing PAD, with a relative risk of 2–4 times that of non-diabetics.10 For every 1% increase in HbA1C in type 2 diabetes, the risk of PAD increases by 28%.14 Diabetics are also much more likely to develop further PAD complications, with a risk of major amputation 7–15 times greater than non-diabetics with PAD. This is likely due to the peripheral sensory neuropathy associated with diabetic foot conditions, meaning that diabetics often present later, as their disease has been asymptomatic. Diabetes also causes rheologic changes, with increased blood coagulability, and a greater predisposition to acute and subacute thrombotic episodes, and increased vascular inflammation with calcification of the intima and more widespread vascular disease.
Figure 65.2 The age-and sex-specific prevalence of PAD (and 95% CI) according to age for men (white bars) and women (shaded bars). (From Meijer et al10, with permission.)
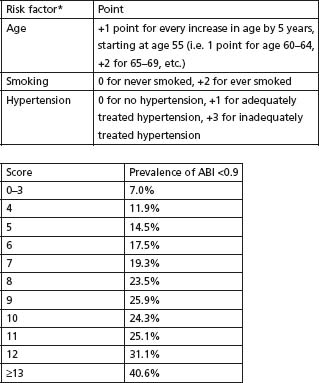
The Framingham investigators have reported that age, sex, cholesterol, hypertension, cigarette smoking, diabetes and pre-existing CAD are associated with increased risk of intermittent claudication, and have developed and published an intermittent claudication risk score based on these risk factors.15 Bendermacher et al16 have also developed a prediction rule to guide general and family practitioners with regard to which patients to screen for PAD, based on age, smoking status and hypertension (Fig. 65.3).
Figure 65.3 The PREVALENT Risk Score. * Risk factors were examined using a stepwise multiple logistic regression model in 7454 subjects, which consistently removed gender, diabetes, hypercholesterolaemia, positive family history and body-mass index. (Adapted from Bendermacher et al.16, with permission.)
Despite the prognostic importance of asymptomatic as well as symptomatic PAD, it is underdiagnosed in routine medical practice.17 The initial clinical assessment for PAD is a history and physical examination. A history of intermittent claudication is useful in raising the suspicion of PAD, and symptoms are usually assessed by asking the person about the presence of exercise-induced calf pain, not present at rest, and which is relieved within 10 minutes by rest. The most recent ACC/AHA guidelines for the management of PAD18 recommend a vascular “review of symptoms” in every clinical history, where people are asked routinely about leg symptoms on exertion, rest pain in the lower leg or foot, and the presence of poorly healing leg or foot wounds. However, reliance on symptoms alone sig-nificantly underestimates the true prevalence of PAD. Persons with PAD may have atypical symptoms, or even no symptoms,10,17 and reliance on a classic history of claudication alone for screening purposes will miss 85–90% of PAD diagnoses.17 Persons with PAD are more than twice as likely as those without PAD to have a history of myocardial infarction, angina, congestive heart failure, stroke or transient ischemic attack13 and therefore PAD screening must be considered in these patients.
Physical examination is an important part of the PAD evaluation, and a number of bedside tests can be performed. The most useful positively predictive clinical features for screening asymptomatic persons for PAD are a positive history of claudication (likelihood ratio (LR) for PAD 3.3, 95% confidence interval (CI) 2.3–4.8), listening for a femoral, iliac or popliteal bruit (LR 4.8, 95% CI 2.4–9.5, for the presence of one bruit at rest) and palpation for a pulse abnormality (femoral, popliteal, dorsalis pedis or posterior tibial; LR 3.1, 95% CI 1.4–6.6). For people with symptomatic PAD, clinical features such as cool skin (L 5.9, 95% CI 4.1–8.6), the presence of a bruit (LR 5.6, 95% CI 4.7–6.7, for the presence of one bruit at rest), and a pulse abnormality (LR 4.7, 95% CI 2.2–9.9) are positive predictors of PAD.19 However, reliance on a pulse abnormality alone can overestimate the incidence of PAD.1 A scoring system for PAD risk using arterial Doppler signals has been published20 and is useful for identifying those patients who require further testing with an ABI test. Commonly, patients with intermittent claudication are classified using either the Rutherford or the Fontaine staging approach. The Rutherford staging is currently recommended5 (Table 65.1).
Table 65.1 Classification of peripheral arterial disease: Rutherford categories
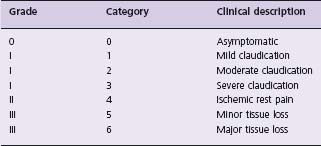
Reproduced with permission from Schmieder FA, Comerota AJ. Am J Cardiol 2001;87(12, suppl 1);3–13.
Ankle Brachial Index
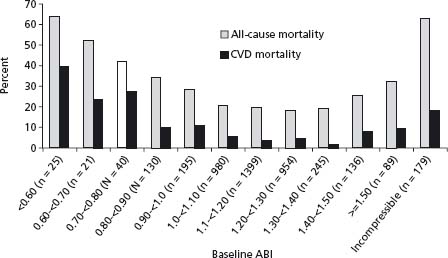
The ABI is a simple bedside test which can be rapidly performed, and which is most often used in epidemiologic studies to assess the presence of PAD (Fig. 65.4). The ABI is useful for both diagnosis and assessment of the efficacy of therapeutic interventions, and has been shown to have a high sensitivity and specificity for PAD, with a sensitivity of +90% and specificity of 95% for detecting angiographically significant disease with an ABI of 0.9 or less.21 An ABI of < 0.9 has typically been used to diagnose PAD in large epidemiologic studies, and is recommended as the cut-off by both the American Heart Association18 and the Transatlantic Inter-Society (TASC) guidelines.5 Furthermore, the presence of a low ABI is highly specific for adverse CV prognosis, although its sensitivity is low. For example, a low ABI (< 0.8–0.9) has a sensitivity and specificity for the prediction of incident CAD of 16.5% and 92.7%, of incident stroke of 16.0% and 92.2%, and of CV mortality of 41.0% and 87.9% respectively.8 The ABI shows a U-shaped relationship with both all-cause and cardiovascular mortality (Fig. 65.5), with both ABIs of < 0.9 and > 1.4 associated with increased risk.22 Higher ABIs > 1.4 are associated with abnormal calcification of the arterial wall and resultant non-compressibility of the vessel, such as is common in diabetes.
Figure 65.4 Measurement of the Ankle Brachial Index. Systolic blood pressure is measured in both brachial arteries, and in the posterior tibial and dorsalis pedis arteries using a Doppler ultrasound device. The higher of the pedal systolic pressures is chosen on each side, and the higher of the two brachial pressures is chosen. The ABI for each side is calculated by dividing the ankle pressure by the arm pressure. (Reproduced with permission from Hiatt WR. N Engl J Med 2001; 344 (21):1608–21.)
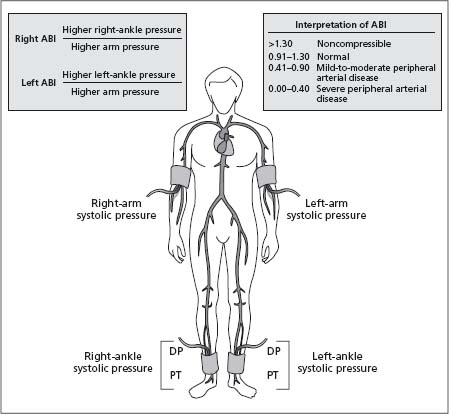
Figure 65.5 All-cause and CVD mortality in a population-based cohort of American Indians, showing the “U” shaped relationship with ABI. (Reproduced from Resnick et al22, with permission.)
Performing an ABI
With the patient in a supine position, the brachial systolic pressure is taken in both arms and the highest pressure recorded. Some recommend that the pressure be recorded ideally with a handheld 5 or 10 mHz Doppler ultrasound17 though use of a stethoscope is acceptable, as long as the methods are consistent over time. Then the systolic pressure at the ankle, in both the anterior tibial and dorsalis pedis arteries, is determined using a 10–12cm sphygmo-manometer cuff around the ankle and the Doppler, and the highest pressure is recorded. The ABI is the ankle pressure divided by the brachial pressure. If the ankle arteries are non-compressible (such as in patients with diabetes mellitus or the very elderly) the ankle toe index can be a more informative test of distal disease, with an index of <0.70 indicating PAD. Some patients, in particular those with iliac disease, may not have a detectable abnormality on the ABI at rest, but complain of symptoms on exercise. The abnormality in these patients may only become apparent on exercise, as the inflow velocity increases, so a treadmill or plantarflexion test may be performed, with the ABI measurement repeated after exercise. Other useful non-invasive testing includes segmental Doppler pressure measurements, which uses a cuff method to identify pressure drops along the lower limbs, to try and isolate the lesion. However, weaknesses of this test are the possibility of missing iliac lesions, and the difficulty in interpreting results in the very old or diabetic subjects.
Treadmill testing
Treadmill testing is particularly useful for identifying PAD in patients in whom the disease is suspected but who have no reduction in ABI at rest. This is sometimes the case in patients with isolated iliac lesions. The procedure requires an initial measurement of either the ABI or full segmental Doppler leg pressures at rest. The patient is then asked to walk (typically on a motorized treadmill with a programmed schedule, such as at 3.2 km/h (2mph), 10–12% grade) for a maximum of five minutes or until maximal claudication pain occurs, unless another reason to stop such as coronary ischemia arises. Following this, the ankle pressure is again measured. A decrease in ABI of 15–20% is diagnostic of PAD. For patients who are uncomfortable using a treadmill, especially older patients, a six-minute walk test along a corridor can also be an effective exercise modality
Diagnostic imaging
This is usually reserved for patients in whom an intervention is being planned or to follow up graft patency. Historically, the gold standard for diagnosis of PAD has been conventional angiography, but because of its invasive and labor-intensive nature and the risks associated with it, non-invasive imaging tools are frequently used.
The primary use of angiography is detection of disease in the arterial tree in persons in whom an intervention is being planned, such as those with critical ischemia or severe intermittent claudication symptoms unresponsive to lifestyle or medical therapy. The advantages of angiography are that definition of the vascular tree is optimized, and hemodynamic parameters may be measured directly. However, as an invasive procedure, angiography carries risks of contrast nephropathy and/or anaphylaxis, access site bleeding or hematoma, atheroembolism, and rarely vascular disruption with dissection or rupture. It is therefore best reserved for carefully chosen patients.
Duplex ultrasound
is used to identify exact lesion sites in persons with symptoms or an abnormal ABI, and also has utility in venous graft surveillance post peripheral bypass procedures. The sensitivity and specificity for detecting lesions are high, reported as 94% by one meta-analysis.23 Duplex ultrasound is especially powerful when using color-guided methods. However, it is labor intensive and requires a high level of technical expertise.
Contrast computed tomographic (CT) angiography
is a further option for imaging the peripheral arterial system. Previously hampered by poorer image quality and slow scanning times, the new generation of multidetector array CT scanners offer high-quality images at high imaging speeds, although image reconstruction can be time consuming. Multidetector row spiral CT angiography (CTA) has been shown to provide sufficient clinical information, although at lower therapeutic confidence, when compared with digital subtraction angiography (DSA). It has excellent sensitivity and specificity for depicting arterial occlusions (sensitivity and specificity of 88.6% and 97.7%) and stenoses of at least 75% (92.2% and 96.8% respectively).24 A meta-analysis of CTA compared with DSA showed sensitivity and specificity of detecting a stenosis >50% for CTA of 92% and 93%, respectively.25 Another study randomized patients to either contrast MR angiography or multi-detector row CTA and found similar clinical utilities, but decreased costs in the CTA group.26 However, the limitations of CTA include difficulty in interpretation of images in which there is a high degree of scatter caused by highly calcified vascular segments, the potential for renal impairment due to contrast injections, and the exposure to ionizing radiation.
Contrast-enhanced magnetic resonance (MR) angiography
is an alternative to CTA and has the clear advantage of providing a “road map” of the arterial system of the lower limbs. Again, it is operator dependent, of limited use in patients with a contrast allergy, pacemaker or cardiac stent, and is expensive. Nevertheless, it has been shown to have better diagnostic accuracy than duplex US and to have similar accuracy to DSA, with sensitivity and specificity of greater than 90% quoted for stenoses of > 50% in studies using three-dimensional gadolinium-enhanced methods.27
Therapy for PAD can be divided into general lifestyle measures, measures aimed at improving symptoms, and measures to optimize secondary prevention of CV events. Therapies mentioned will be graded as per the Evidence Based Cardiology grading system. We will also discuss emerging therapies and the evidence for these to date.
Lifestyle measures
Smoking cessation
Our understanding of the effects of smoking cessation in PAD patients comes primarily from observational studies, as there have been no prospective randomized controlled trials (RCTs) of smoking cessation and its effects on CVD outcomes in patients with PAD. Nevertheless, reports show that smoking cessation is associated with improvements in claudication symptoms, reduced rest pain, improved graft patency rates post lower limb bypass procedures, and decreased risk of death, MI or amputation. Patients who have undergone lower extremity bypass surgery also have a higher rate of major amputation if they are heavy smokers (>15 cigarettes per day) compared with more moderate smokers (21% amputation rate vs 2%, P < 0.001).28 Smoking cessation rates have also been associated with reduced mortality in an RCT of small infrarenal aneurysm outcomes,29 where the mortality benefit in the surgical vs surveillance group could be explained by the 12-fold increase in the odds of smoking cessation in the surgical group.30
Smoking cessation can be difficult to achieve, but effective interventions include physician guidance (2% of patients advised by their physician to quit had remained off cigarettes at one year31) and cessation aids including nicotine replacement therapy, varenicline (Champix ™), bupropion, and other antidepressant therapies.
Simple physician advice to quit smoking in a brief clinical encounter has been shown to increase quit rates by 5%.32 Nicotine replacement therapy (NRT) has been shown to be up to 13% effective in achieving adherence to non-smoking at one year,31 with a meta-analysis of data from 16 RCTs of transdermal NRT patches reporting an odds ratio (OR) for 12-month abstinence of 1.75 (95% CI 1.49-2.05).33 An increase in success for smoking cessation of 50-70% with all types of NRT was reported in a Cochrane review and meta-analysis of 132 trials by Stead et al.34 Bupropion (Zyban ™), an atypical antidepressant agent, has been found to be superior to placebo and NRT, with 12-month abstinence rates of 30%, although there was a high withdrawal rate from therapy (11%) and an unusually high quit rate in the placebo group.35 A second RCT also found that bupropion doubled the success of smoking cessation, with an odds ratio of abstinence at 12 months for bupropion of 2.78 (95% CI 1.70–4.63), compared with placebo and usual care.36 However, bupropion should not be used in patients with epilepsy, and only used with great caution in patients with low seizure thresholds.
A newer agent which has recently entered the marketplace is varenicline tartrate (Champix ™), a partial nicotine receptor agonist. A Phase II RCT comparing varenicline to placebo demonstrated significantly higher quit rates of 14.4% with varenicline, compared with 4.9% for placebo, P = 0.002.37 This was followed by a Phase III study which showed continuous abstinence rates for 9–52 weeks were significantly higher among patients receiving varenicline (21.9%) or bupropion (16.1%) compared to placebo (8.4%).38 Varenicline is reasonably well tolerated, with better adherence rates than for bupropion, although larger and longer term trials of varenicline are needed.
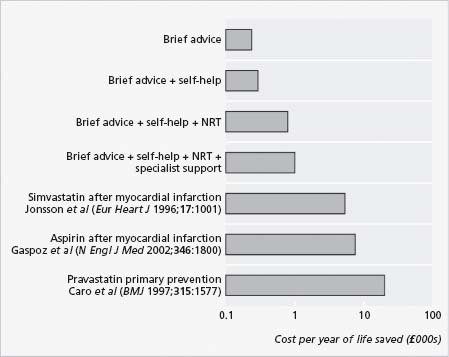
Smoking cessation aids have been shown to be cost effective, with cost per life-year saved less for smoking cessation aids than for other secondary prevention steps commonly used post myocardial infarction (Fig. 65.6).
Figure 65.6 Cost effectiveness of smoking cessation strategies post MI when compared with other routine secondary prevention methods. (Reproduced with permission from Parrott S, Godfrey C. BMJ 2004; 328:947–9.)
Recommendations
- Smoking cessation in all patients, to reduce PAD symptoms and future adverse CVD events (Class 1, Level C1).
- Use of smoking cessation aids such as physician advice, NRT, bupropion or varenicline to aid in quitting (Class 1, Level B).
Exercise
Patients with PAD benefit from a regular exercise program to optimize walking ability and maximal walking time.39 A meta-analysis of 21 trials of walking therapy found that walking to near maximal pain, for 30 minutes and as partf a program lasting more than six months, increased the distance to onset of claudication pain by 179% (225.3 m) and the distance to maximal claudication pain by 122% (397.5m).40 Resistance training is not as beneficial as regular walking to near maximal pain threshold for 30 minutes at least four times a week, and supervised programs have been shown to be more beneficial than non-supervised, although there are nevertheless benefits to be gained from the home-based programs. There is also preliminary evidence from two small RCTs (n = 3541 and n = 6742) which suggests that arm ergonometry may be as successful as treadmill training in increasing initial claudication distance and maximal walking distance. This may offer an exercise alternative to those with severely reduced mobility.
Although one theory is that walking induces angiogenesis and collateral growth around the occluded or stenosed area, it is actually more likely that the benefits are due to improvements in muscle tone with muscle hypertrophy, improved endothelial function and altered gait.18 It was also shown in one trial that exercise therapy was more beneficial for walking times than percutaneous revascularization therapies43 although this has not been replicated elsewhere. Exercise training for patients with PAD is optimally supervised by an exercise physiologist, physical therapist or nurse, in the setting of a cardiac rehabilitation program or similar,18 with the option of further non-invasive monitoring, since these patients may have concomitant cardiac disease. Supervised exercise sessions are typically scheduled three times a week, for a period of three months, with 30–60-minute sessions each time.5 Exercise also has further benefits in that it can improve dyslip-idemia, hypertension and risk of type 2 diabetes mellitus, as well as reducing overall adiposity and Body Mass Index.
Recommendations
- A graded supervised exercise program should be advised to improve walking distance and other cardiovascular risk factors in patients with PAD (Class 1, Level B).
- There is less evidence to support home-based exercise programs (Class IIa, Level B).
- Arm ergonometry may be an acceptable alternative to walking-based programs (Class IIa, Level B).
Therapies for secondary event prevention
As discussed, patients with PAD are at very high risk of adverse vascular events elsewhere in the arterial tree, such as MI and stroke. Diabetes, hypertension and elevated cholesterol are not only etiologic factors in the pathogenesis of PAD, they also contribute to high rates of CV events among patients with established PAD. Therefore, it is generally accepted that control of etiologic risk factors will result in improved cardiovascular prognosis. Below we present evidence to support this practice.
Glycemic control in patients with diabetes
Control of blood glucose is advisable in patients with diabetes, to reduce the risk of adverse CVD events. The Steno-2 trial44 randomized 160 type 2 diabetes patients to conventional or intensive control with a target HBA1C of < 6.5%, in conjunction with tight blood pressure and lipid control with a mean follow-up of 13.3 years. It reported reduced risks of cardiovascular mortality (hazard ratio (HR) 0.43, 95% CI 0.19–0.94, P = 0.04) and of cardiovascular events (HR 0.41, 95% CI 0.25–0.67, P < 0.001) with intensive glycemic control. However, a conflicting message has been given by the ACCORD study, in which an increase in total mortality was noted in the intensive control intervention arm, which aimed to reduce HBA1C in type 2 diabetes patients to <6.4%.45
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


