13
Peripheral and abdominal aortic aneurysms
Definition of an aneurysm
Derived from the Greek word aneurysma describing ‘a widening’, an arterial aneurysm is defined by an increased vessel diameter of 50% or more than that of the non-dilated adjacent vessel.1
Prevalence of arterial aneurysms
Population screening studies indicate that the prevalence of abdominal aortic aneurysm (AAA) increases with age,2,3 occurring in 7–8% of men over 65 years.3,4 The disease prevalence is six times higher in men than in women,5 with AAA rupture the seventh most common cause of male death in the UK. Between 1951 and 1995 there was a steady increase in age-standardised deaths from all aortic aneurysms in men, from 2 to 56 per 100 000 population in England and Wales. More recently, data suggest that the incidence of abdominal aortic aneurysm (AAA) may now be declining. Between 1997 and 2009 there has been a reduction in age-adjusted mortality from AAA from 40.4 to 25.7 per 100 000 population for England and Wales and from 30.1 to 20.8 per 100 000 population in Scotland.6
There is considerably less information about the prevalence of peripheral aneurysms, although it is recognised that these frequently occur in association with AAA. Approximately 25% of patients with AAA have coexisting femoral or popliteal aneurysms.7 It is likely therefore that peripheral aneurysms share a common aetiology with AAAs and that changes in their prevalence match those of aortic aneurysms.
Pathogenesis of aortic aneurysms
Metabolic regulation of both elastin and collagen proteins in the aortic wall is under the control of several enzymatic agents. The most important group of these mediators appears to belong to the zinc- and calcium-requiring matrix-degrading metalloproteinases (MMPs), and there is compelling evidence that abnormal local MMP production and regulation is associated with the pathogenesis of aortic aneurysms. The elastolytic subtypes MMP-9 and MMP-2 appear to be the most influential in AAA pathogenesis.8,9 A chronic inflammatory infiltrate composed of T cells, macrophages, B lymphocytes and plasma cells is a typical histological feature of AAA. Although the antecedent trigger for this cellular migration remains unclear, much of the subsequent vessel wall destruction appears to be mediated by the cytokines and chemokines released by the infiltrate with induction and activation of MMP species.10
Although certain phenotypes have been associated with increased frequency of disease progression, as yet no single genetic anomaly or polymorphism has been universally identified within all AAA patients. This approach to pathogenesis may eventually form the basis of genetic testing of specific increased-risk populations, allowing more focused surveillance and early intervention.8
In an effort to unify all aspects of this complex process, Ailawadi et al.9 have proposed a model of aortic aneurysm pathogenesis. They postulate that the initial trigger for AAA may be a combination of factors such as fragmented medial proteins, localised haemodynamic stress or a genetic predisposition that causes inflammatory cells to migrate into the aortic wall. This inflammatory infiltrate is rich in cytokines, chemokines and reactive oxygen species, and attracts further cellular influx with expression and activation of proteases, in particular those of the MMP group. Subsequent unregulated connective tissue turnover results in medial degeneration of the aorta and aneurysmal dilatation. The proteolysis is exacerbated by the inherent increase in wall stress with progressive AAA expansion. If untreated, the sequence cascades, with eventual aortic rupture.10
Infrarenal abdominal aortic aneurysms
Most AAAs remain asymptomatic until rupture, with approximately 75% being symptom free at diagnosis. The majority of these cases are detected as an incidental finding during the course of investigation of unrelated cause. Aetiological factors include increasing age, male sex, ethnic origin, family history, smoking, hypercholesterolaemia, hypertension and prior vascular disease. Of these, male sex and smoking are the most important, increasing the chances of AAA development by 4.5 and 5.6 times, respectively.11
Symptomatic and ruptured AAAs
Rapid expansion (>1 cm/year) or the development of symptoms such as abdominal pain, tenderness and back pain are usually an indication for prompt surgical intervention, irrespective of size. This is because of a higher rupture rate.12
Inflammatory abdominal aortic aneurysms (IAAAs)
IAAAs account for 3–10% of all AAAs and were not classified per se until the early 1970s.13 Classical defining features are the triad of a thickened aneurysmal wall, marked perianeurysmal/retroperitoneal fibrosis and dense neighbouring visceral adhesions. Abdominal or back pain, weight loss and an elevated erythrocyte sedimentation rate in a patient with known aortic aneurysm confer a diagnosis of IAAA until proven otherwise.14
Population screening for AAAs
B-mode ultrasound scanning using portable equipment has been shown to be effective in detecting AAAs and is inexpensive.15 Furthermore, it has been estimated that a single ultrasound scan in males of 65 years of age would detect 90% of aneurysms at risk of rupture.16
Principles of AAA management
The fundamental principle underpinning AAA management strategy is the prevention of rupture. The role of medical therapy is important and includes blood pressure control, cholesterol reduction, antiplatelet therapy and smoking cessation. AAA size is still considered the most important factor in prediction of rupture. From a meta-analysis of 13 studies, Law et al. have quantified this annual risk for differing initial size19 (Table 13.1).
Table 13.1
Annual AAA rupture risk in relation to size
| AAA size (cm) | Risk of rupture per year (%) |
| < 3.0 | 0 |
| 3–3.9 | 0.4 |
| 4–4.9 | 1.1 |
| 5–5.9 | 3.3 |
| 6–6.9 | 9.4 |
| 7–7.9 | 24 |
Adapted from Law MR, Morris J, Wald NJ. Screening for abdominal aortic aneurysms. J Med Screening 1994; 1:110–15.
The UK Small Aneurysm Trial and US Aneurysm Detection and Management (ADAM) trials were designed to provide guidelines as to when to offer elective surgery on the basis of aneurysm size.12,20
AAA repair
Investigation of the patient with known AAA
The aims of evaluation of patients diagnosed with an AAA are threefold:
1. to identify patients in whom the balance of risk favours operative intervention;
2. to reduce perioperative morbidity and mortality by identifying patients who may require further investigation or treatment of comorbidity prior to surgery;
3. to assess the anatomical suitability of the aneurysm for open or endovascular repair.
Accurate clinical assessment is imperative as it is recognised that perioperative mortality is related to the pre-existing physiological status of the patient.20 The majority of early deaths following AAA repair are related to cardiac events and if pre-existing cardiac abnormalities are detected and treated prior to surgery, a substantial improvement in survival rates can potentially be achieved.21 Respiratory complications are the most common form of morbidity after major abdominal surgery and occur after 25–50% of all such operations, including aortic aneurysm repair.22 The risk of perioperative renal failure is increased in those with pre-existing renal disease, diabetes or coexisting cardiac disease, and in those aged over 60 years.
Elective open AAA repair
Although elective operative blood loss is usually minimal, excessive bleeding can occasionally be encountered either from back-bleeding lumbar arteries following opening of the sac or from the anastomotic suture lines. The routine use of a cell saver to preserve the patient’s own red cells is a useful adjunct under these circumstances.24
Minimally invasive open AAA repair
The advent of endovascular techniques has stimulated interest in developing other less invasive alternatives to conventional open surgery. These include shorter (6-cm) incisions and totally laparoscopic techniques.25 Custom-made retractors and other instrumentation have been developed for these procedures. It is claimed that surgical trauma is reduced significantly, with benefits in terms of lower operative mortality and morbidity rates and more rapid recovery of the patients. However, to date, reliable comparative data are lacking.
Emergency open AAA repair
Survival following AAA rupture is poor in patients who have suffered a cardiac arrest, in the very elderly and in those who remain persistently unconscious. A decision not to offer surgical intervention to such patients is justified. Low or absent urinary output should not of itself be a contraindication to surgery, but its consideration within risk-scoring systems such as the Glasgow Aneurysm Score (see later) may help with appropriate patient selection for surgery.26
Outcome following open surgical AAA repair
Factors associated with a poorer outcome following open AAA repair include increased patient age, larger aneurysm size and the presence of preoperative renal failure.28,29 In order to assist in the prediction of patients at high risk of perioperative mortality and morbidity after elective and ruptured AAA repair, the Glasgow Aneurysm Score (GAS) has been described.26,30 In addition to the patient’s age, differentially weighted patient-specific variables including the presence of cardiac, cerebrovascular and/or renal comorbidity with/without hypovolaemic shock are summed to yield a numerical value that can be extrapolated to a risk bracket (i.e. GAS = age ± cardiac disease (7 points) ± renal dysfunction (14 points) ± cerebrovascular disease (10 points) ± shock (17 points)). Naturally, the GAS alone should not dictate clinical practice, but it can serve as a useful adjunct for risk stratification in assessing a patient’s suitability for AAA surgery.
Patients with AAAs have a markedly decreased life expectancy in comparison with age- and sex-matched control populations. The 5-year survival of patients postsurgery varies from 62% to 72% (compared with 83–90% in age- and sex-matched populations), with the majority of deaths due to coronary artery disease.21,28 Quality-of-life studies have shown an improved perception of general health in the first 2 years after open repair in comparison with patients who are under surveillance.32
The UK Small Aneurysm Trial showed that only about 25% of patients with ruptured aneurysms make it to theatre for emergency repair.12 A subsequent meta-analysis showed that there has been a gradual improvement in survival following surgery for ruptured AAAs over the last 40 years of the order of 3.5% per decade. However, this study also showed that the estimate of operative mortality rate remains high at approximately 41%.33
Endovascular AAA repair (EVAR)
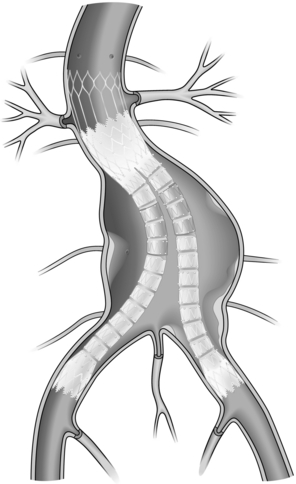
Since the first case of EVAR was reported by Parodi et al. in 1991,34 this minimally invasive technique has become increasingly popular with both physicians and patients alike. The fundamental goal of EVAR is sustained aneurysm exclusion from the systemic circulation by means of a preoperatively sized stent graft, preventing further aneurysm expansion and therefore eliminating rupture risk (Fig. 13.1).
Indications and eligibility for EVAR: Originally developed as a treatment option for those where existing comorbidity prohibited open surgical repair, the current indications for EVAR are less clear. The approach may well be preferable in cases of a hostile abdomen (e.g. peritoneal adhesions, intestinal stomas) and also where the risk of iatrogenic injury is significant (e.g. IAAA repair).35 In the absence of such factors and in fitter patients the role of EVAR remains in dispute.
Contrary to open AAA repair, EVAR suitability depends not only on patient fitness but also aneurysmal morphology. Limitations of contemporary endografts and their delivery platforms continue to preclude EVAR in many patients, with current elective eligibility rates quoted between 55% and 74%.36,37 Features promoting EVAR suitability include a healthy proximal neck with limited angulation, at least 15 mm in length, no more than 30 mm in diameter and with smooth, parallel, endoluminal surfaces without significant mural thrombus. In addition the iliac arteries should be of sufficient calibre, at least 7 mm for most devices, to facilitate the passage of the delivery apparatus into the abdominal aorta. Short ectatic common iliac arteries represent relatively unfavourable anatomy for EVAR in view of the need for a reliable distal seal.37,38
EVAR devices: Four distinct generic schemes are currently available for EVAR of infrarenal AAAs: straight aorto-aortic tube endografts, bifurcated systems, aorto-mono-iliac systems and combined bifurcated and iliac branched stent grafts. All devices form their proximal seal within the infrarenal aortic segment but differences exist in the location of the distal ‘landing site’. The pioneering aorto-aortic straight stent graft resides entirely in the abdominal aorta but is only suitable for a very limited number of cases. The published early experience of EVAR showed that where aorto-aortic tube endografts were used for fusiform aortic aneurysms, an unacceptably high incidence of late device failure occurred due to extension of the pathological process into the distal neck and aorto-iliac segments.39 There is still, however, a place for such tube grafts in localised saccular aneurysms, postoperative pseudoaneurysms and penetrating aortic ulcers.
Bifurcated systems offer the best solution by providing the potential for distal fixation beyond the vascular segments most likely to suffer further aneurysmal expansion in the long term, while maintaining normal anatomical relations. The currently available standard bifurcated devices may be appropriate for use in up to 50% of patients,40 although this figure is increasing as newer devices become available for the treatment of short and angulated proximal necks. The remainder of EVAR-eligible aneurysms with more challenging anatomy (including those with aorto-iliac aneurysms) require the use of branched stent grafts or the aorto-mono-iliac stent graft.41 Aorto-mono-iliac endografts require an extra-anatomical (femoro-femoral) crossover graft for maintenance of contralateral lower limb blood supply, following endoluminal plugging of the contralateral common iliac artery. Iliac branched stent grafts are a relatively new and unproven development but provide an additional option for younger patients with bilateral aorto-iliac aneurysms.
Patient assessment and EVAR technique: The patient should be formally assessed and prepared for EVAR as if for conventional open surgery. Detailed vascular imaging (preferably MD-CTA) should be obtained to enable calibration of the entire abdominal aorta and iliofemoral segments in order to enable graft sizing and provide information regarding arterial access. Informed consent should include the routine morbidity and also the known EVAR-specific complications, including contrast nephropathy, endoleak (see later) and open surgical conversion. Ideally, the theatre should be designed for combined interventional/operative procedures and equipped with a C-arm or equivalent for intraoperative imaging.
After anaesthetic induction the patient is appropriately positioned, prepped and draped. The procedure usually commences by surgical cut-down to the femoral artery to gain access to the arterial circulation, although some advocate a percutaneous approach42,43 assisted by the increasing usage of femoral closure devices.44 After femoral access is achieved, a soft wire and catheter are placed into the suprarenal aorta and a stiff guidewire is introduced through the catheter. Stiff guidewires are not intended to be ‘working’ wires and it is not sensible to try to negotiate tortuous iliac vessels with them. The stent graft body is introduced over the stiff guidewire and the renal arteries are imaged. The image intensifier should be angled to optimise the view of the renal arteries and this typically requires a small amount of cranio-caudal and oblique tilt.
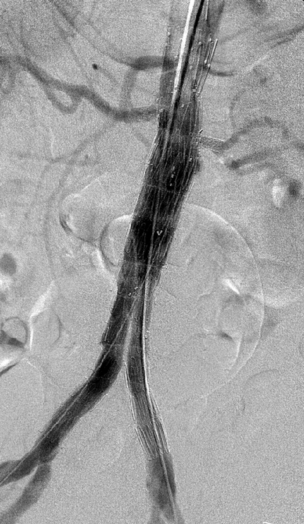
Completion angiography is performed to determine whether the aneurysm has been excluded and to ensure that there has been no encroachment by the fabric of the graft on the orifices of the visceral or internal iliac arteries (Fig. 13.2). Every effort must be made to resolve all primary type I endoleak before the patient is allowed to leave the operating room.
EVAR-related complications and device failure: The physiological advantages of EVAR are reflected by the reduced requirement of postoperative critical care support and incidence of significant cardiac, pulmonary and renal complications. However, in addition to these routine causes of postoperative morbidity following AAA repair, EVAR unfortunately carries with it a distinct spectrum of its own specific complications.
Endoleak: Endoleak is defined as the persistence of blood flow outside the lumen of an endovascular graft but within an aneurysm sac or the adjacent vascular segment being treated by the stent.45 The leak may be described as primary, originating at the time of EVAR, or secondary, referring to a leak not seen at completion angiography but demonstrated on subsequent imaging. Endoleaks have been classified according to the source of aberrant blood flow, since this characterises the endoleak and hence the potential for deleterious sequelae (Table 13.2).46
Table 13.2
| Endoleak type | Source | |
| I | A: Proximal | Graft attachment site |
| B: Distal | ||
| C: Iliac occluder | ||
| II | A: Simple (single vessel) | Collateral vessel |
| B: Complex (>2 vessels) | ||
| III | A: Junctional leak | Graft failure |
| B: Mid-graft hole | ||
| C: Other (e.g. suture hole) | ||
| IV | Graft wall porosity | |
| V | A: Without endoleak | Endotension |
| B: With sealed endoleak | ||
| C: With type I or III leak | ||
| D: With type II leak |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree