Perioperative Anesthetic Considerations in Noncardiac Surgery for Those at High Risk of Cardiovascular Disease
Michael F. Roizen
John Ellis
Srinivas Mantha
Joe Foss
Goals of Perioperative Management
The goals of anesthesia for those at risk of or with overt cardiovascular disease are similar for any procedure: to minimize patient morbidity and maximize surgical benefit. These goals should be achieved in the most cost-effective manner. The increasing age of the population in Western societies and the desire to restore functional status will likely increase the number of operative procedures performed in the elderly and consequently those with likely significant cardiovascular disease.
The changes in outcome after vascular procedures illustrate the magnitude of improvements in perioperative care in the last 40 years. Perioperative morbidity from such procedures has decreased dramatically, from a 6-day mortality of greater than 25% for major aortic reconstruction in the mid-1960s to less than 2.5% 6-day mortality today. We believe that advances in preoperative preparation and anesthetic management are responsible for a substantial portion of these beneficial changes (1,2,3). The anesthesiologist may have a greater influence in reducing the morbidity and costs of vascular surgery than in any other complex surgical procedure through the ability to affect outcome and resource utilization.
Nowhere in medicine is the team performance and function likely to be more important to patient outcome in complex surgery than with the combination of primary care physician; cardiologist; surgeon; anesthesiologist; critical care, pain therapy, and rehabilitation subspecialists; and nurse. Why do we specify complex surgery? Because the data are clear: advances in perioperative care have made anesthesia for minimally invasive procedures as safe as the other events in daily living encountered by such patients (4,5). This chapter intends to inform all team members of the perspectives and concerns of the anesthesiologist that bear on perioperative medical and surgical management in care of patients about to undergo complex surgery (we use anesthesia considerations for vascular procedures as the model because these are the best characterized).
The heart is the major focus of the anesthesiologist’s attention in such patients because myocardial dysfunction is the most important cause of morbidity after vascular and other complex surgery in the elderly (1,2). Recent studies have identified and emphasized that improvements in outcome were due initially to improvements in fluid management and now
to the refinement of preoperative risk-stratification and risk-reducing strategies and the management of the consequences of the stressful nature of the postoperative period to patients. These strategies protect the cardiovascular system and also help to preserve other organ systems (particularly renal and central nervous systems). Thus this chapter reviews current controversies in the selection of anesthetic techniques, monitoring modalities, guidelines (6), chronic medical therapy initiation and continuation, and organ protection strategies.
to the refinement of preoperative risk-stratification and risk-reducing strategies and the management of the consequences of the stressful nature of the postoperative period to patients. These strategies protect the cardiovascular system and also help to preserve other organ systems (particularly renal and central nervous systems). Thus this chapter reviews current controversies in the selection of anesthetic techniques, monitoring modalities, guidelines (6), chronic medical therapy initiation and continuation, and organ protection strategies.
Aspects of Atherosclerosis Important to Perioperative Considerations
Advances in the understanding of factors in the pathogenesis of atherosclerosis enhance the ability to assess and minimize risk in the individual patient. The realization that atherosclerosis is a generalized inflammatory disorder of the arterial tree with associated endothelial dysfunction (7) leads to very important risk reduction assessments and strategies.
The predisposing risk factors for atherosclerosis have been classified as “old,” “old/new,” and “new” (Table 41.1) (8). The metabolic syndrome (9) and the six situations that contribute to the proinflammatory, prothrombotic state (10) each can be modified preoperatively. The components of the metabolic syndrome constitute a particular combination of what the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) terms underlying, major, and emerging risk factors. According to ATP III, underlying risk factors for cardiovascular disease are obesity (especially abdominal obesity), physical inactivity, and atherogenic diet; the major risk factors are cigarette smoking, hypertension, elevated low-density-lipoprotein cholesterol (LDL-C), low high-density lipoprotein (HDL), family history of premature coronary heart disease, and aging; and the emerging risk factors include elevated triglycerides, small LDL particles, insulin resistance, and glucose intolerance. These might seem to require long-term treatment, but each can be modified even in as short a period as 2 weeks before surgery. Furthermore, even the hypertension- and stress-reducing therapies proposed by the Joint Commission on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) such as aiming at a blood pressure of 115/75 mm Hg lead to different perioperative strategies and tactics (11).
These understandings of various risk factors in the pathogenesis of atherosclerosis have enhanced our ability to assess long-term and perioperative risk in the individual patient. For example, a simple scoring system for calculating a 10-year risk of acute coronary events (12) influences assessment of perioperative management. The model incorporates the following eight independent risk variables ranked in order of importance: age, LDL cholesterol, smoking, HDL cholesterol, systolic blood pressure, family history of premature myocardial infarction (MI), diabetes mellitus, and triglycerides.
TABLE 41.1 Risk Factors for Atherosclerosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
The natural history of cardiovascular disease causes further changes in therapies. Persons with cerebral atherosclerosis are at increased risk for ischemic stroke. Stroke is the third-leading cause of death and principal cause of long-term disability in the United States, with 600,000 new or recurrent strokes occurring annually. Presence of even asymptomatic carotid artery disease identifies patients at risk for fatal and nonfatal MI especially in the perioperative period. The prevalence of greater than 25% carotid stenosis in patients older than 65 years was 43% in men and 34% in women in one of the Framingham studies. Those with renal artery atherosclerosis are at risk for perioperative renal failure. Moreover, once disease is apparent in one vascular territory, there is increased risk for adverse events in other territories. For example, patients with peripheral arterial disease (PAD) have a fourfold greater risk of myocardial infarction and a two- to threefold greater risk of stroke than patients without PAD. Regardless of whether symptoms are evident, patients with PAD are six times more likely to die within 10 years than patients without PAD. Patients with symptomatic PAD have a 15-year accrued survival rate of approximately 22% compared with a survival rate of 78% in patients without PAD symptoms. Current U.S. disease-based data sets have been used to compare 5-year mortality rates from common malignancies with the rate of PAD, providing a “commonsense” yardstick of relative risk. This analysis demonstrated that the patient survival rate for PAD is worse than the outcome for breast cancer and Hodgkin disease.
Given the high morbidity and mortality related to PAD and its perioperative prognostic significance, several methods have been proposed for the detection of PAD. Among the several methods, use of the ankle–brachial index (ABI) is a very popular, simple, noninvasive, and inexpensive measurement for assessing the patency of the lower-extremity arterial system. The ABI is measured by having the patient lie in the supine position and then taking the ankle and brachial blood pressure measurements using a 5- to 7-MHz handheld Doppler device. In the lower extremity, posterior tibial and dorsalis pedis artery systolic pressures are measured and compared with the arm pressure. The ABI is calculated by dividing the higher of the ankle systolic pressures by the higher of two systolic brachial
pressures. Measuring the ABI is the most effective, accurate, and practical method of PAD detection. An ABI value of 1.0 or greater is considered normal, whereas a value less than 0.9 indicates the presence of PAD and approaches 95% sensitivity in detecting angiogram-positive disease. It is almost 100% specific in excluding healthy individuals. The ABI also helps to evaluate the severity of the disease. For example, ABI values of 0.81 to 0.9 are consistent with mild disease and values of 0.5 to 0.81 with moderate disease, and values less than 0.5 are considered to indicate severe obstructive disease. Furthermore, a value less than 0.9 is highly predictive of morbidity and mortality from cardiovascular events linked with PAD. The major disadvantage of the ABI is that some elderly and diabetic patients have calcified arteries that prevent occlusion of blood flow by the blood pressure cuff, which may result in an unusually high ABI reading (>1.50). More recently, large observational studies and atherosclerosis regression trials of lipid-modifying pharmacotherapy have established that intima–media thickness of the carotid and femoral arteries, as measured noninvasively by B-mode ultrasound, is a valid surrogate marker for the progression of atherosclerotic disease (7).
pressures. Measuring the ABI is the most effective, accurate, and practical method of PAD detection. An ABI value of 1.0 or greater is considered normal, whereas a value less than 0.9 indicates the presence of PAD and approaches 95% sensitivity in detecting angiogram-positive disease. It is almost 100% specific in excluding healthy individuals. The ABI also helps to evaluate the severity of the disease. For example, ABI values of 0.81 to 0.9 are consistent with mild disease and values of 0.5 to 0.81 with moderate disease, and values less than 0.5 are considered to indicate severe obstructive disease. Furthermore, a value less than 0.9 is highly predictive of morbidity and mortality from cardiovascular events linked with PAD. The major disadvantage of the ABI is that some elderly and diabetic patients have calcified arteries that prevent occlusion of blood flow by the blood pressure cuff, which may result in an unusually high ABI reading (>1.50). More recently, large observational studies and atherosclerosis regression trials of lipid-modifying pharmacotherapy have established that intima–media thickness of the carotid and femoral arteries, as measured noninvasively by B-mode ultrasound, is a valid surrogate marker for the progression of atherosclerotic disease (7).
Considerations for Vascular and Other Complex Surgery Patients
The goals of vascular surgery are to provide an enduring restoration of normal perfusion so as to prevent stroke, improve functional status, and prevent death from aneurysm rupture. Perioperative management is important because approximately 700,000 patients in the United States underwent vascular surgical procedures in 2002. Increasingly, randomized clinical trials are demonstrating the benefits to patient quality and length of life from revascularization procedures of the aorta, the carotids, the renal arteries, and the lower-extremity arteries (7,13). An explosive growth of less invasive procedures is changing surgery in the elderly, including vascular surgery. New treatment options include lasers, angioplasty, stenting, atherectomy (rotary and directional devices), gene therapy to help prevent restenosis and to promote angiogenesis, and thrombolysis, at times with concomitant or secondary surgery. Although outcome studies for many of these procedures just now are being completed, they appear to be associated with less short-term risk than traditional surgery. Less invasive approaches will invariably lead to procedures being performed in patients with such severe comorbidities that they would previously have been denied surgery. A recent randomized clinical trial involving 60 patients who underwent percutaneous transluminal coronary angioplasty (PTCA) for acute MI showed that intracoronary transfer of autologous bone marrow cells resulted in improvement of the left ventricular systolic function at 6 months’ follow-up. As with this potential application, other developments are both promising and challenging, such as creation of a long-lasting arterial substitute for small-caliber vessels and performance of revascularization less invasively and with less morbidity.
Abdominal aortic aneurysms (AAAs) occur in up to 5% of men older than 65 years of age. Most AAAs are small and require only infrequent follow-up. There are two therapeutic options for AAA patients with lesions large enough to cause worry about rupture: open surgery and endovascular aortic aneurysm repair (EVAR) (3). The main impetus behind EVAR has been its potential for significantly reducing procedural mortality and morbidity, and it was expected to speed recovery and reduce cost through decreased use of hospital resources. The new technology has evoked a mixed response, with enthusiasts and detractors debating its value; unfortunately, bias and conflict of interest may be involved. EVAR seems to be an appropriate elective treatment in patients with AAAs with significant comorbid conditions and suitable anatomy and in patients with relatively limited life expectancy and larger or enlarging AAAs. A recent decision-analysis model suggests that EVAR is preferable in older patients at higher operative risk and open surgery is preferred in younger patients at low operative risk. For atherosclerotic disease of carotid arteries, studies have clearly shown an advantage for surgical endarterectomy compared to medical therapy. Carotid endarterectomy (CEA) is appropriate for symptomatic patients (transient ischemic attacks or nondisabling stroke) with 70% to 99% carotid stenosis and in selected asymptomatic patients with 60% or greater stenosis after careful consideration of additional risk factors. In experienced hands CEA and carotid stenting may have equivalent success and complication rates. Combined perioperative mortality and major morbidity rates should be less than 6% for symptomatic patients and less than 3% for asymptomatic patients for percutaneous procedures, as for CEA. Patients with restenosis after CEA and those with radiation-induced stenosis are at increased risk from surgery and thus good candidates for stenting.
Perioperative management has taken lessons from medical therapy for atherosclerotic vascular disease (AVD) in improving functional status, preventing stroke, preventing limb loss, and reducing potential atherosclerotic progression and cardiovascular morbidity. In general, medical measures typically include smoking cessation, lifestyle changes, antilipidemic therapy, control of blood pressure, control of diabetes mellitus, and antiplatelet therapy. Cessation of smoking is by far the most effective “medical” therapy. Although acute smoking cessation before surgery may not reduce perioperative respiratory complications, patients should be encouraged to stop smoking. Cessation rates are approximately 25% after major surgery. Despite the low success rates, the benefits of smoking cessation are so great that such perioperative programs are probably very cost-effective.
Lifestyle changes such as weight loss and exercise reduce perioperative complications in small randomized and nonrandomized studies. Nonlipid properties can be observed earlier (as early as 2 weeks of therapy) than lipid effects. Patients who were already taking statins when they presented to the hospital with acute coronary events and continued to receive them were less likely to experience more adverse complications or die than patients who never received statins (14). Statin use is also associated with improved graft patency and limb salvage and decreased amputation rate in patients undergoing infrainguinal bypass for AVD (15). Recently, angiotensin-converting-enzyme (ACE inhibitors) were shown to upregulate type III collagen synthesis, which could improve atherosclerotic plaque stabilization (16). Such action may explain the beneficial effects of ACE inhibitors with regard to acute vascular events (e.g., stroke) that are independent of their antihypertensive effect. For example, ACE inhibitor use was also associated with decreased long-term mortality in patients undergoing infrainguinal bypass for AVD (15).
Considerations in Management of Chronic and Perioperative Risk-Reducing Medical Therapies
Antiplatelet therapy is a mainstay of medical therapy for peripheral vascular disease. Chronic therapy with aspirin or other antiinflammatory drugs may retard the progression of atherosclerosis and prevent morbid cardiovascular events.
Therefore, many patients presenting for vascular surgery will be taking aspirin, nonsteroidal antiinflammatory drugs (NSAIDs), and even COX-2 inhibitors, ticlopidine, or clopidogrel. Clopidogrel irreversibly inhibits adenosine diphosphate (ADP)-induced platelet aggregation and reduces formation of both arterial and venous thrombi. Oral platelet glycoprotein IIb/IIIa inhibitors may also be used acutely during percutaneous coronary intervention and as adjunctive treatment of acute coronary syndromes. Considerations of the adverse effects of aspirin, including increased bleeding tendency, gastritis, and renal vasoconstriction, as well as thrombotic thrombocytopenic purpura and neutropenia from ticlopidine must be weighed against potential benefits. In general, we recommend that patients continue to take aspirin through the day of surgery for all non–closed space, non–plastic surgical procedures. Other acute anticoagulant issues are addressed later (17,18).
Therefore, many patients presenting for vascular surgery will be taking aspirin, nonsteroidal antiinflammatory drugs (NSAIDs), and even COX-2 inhibitors, ticlopidine, or clopidogrel. Clopidogrel irreversibly inhibits adenosine diphosphate (ADP)-induced platelet aggregation and reduces formation of both arterial and venous thrombi. Oral platelet glycoprotein IIb/IIIa inhibitors may also be used acutely during percutaneous coronary intervention and as adjunctive treatment of acute coronary syndromes. Considerations of the adverse effects of aspirin, including increased bleeding tendency, gastritis, and renal vasoconstriction, as well as thrombotic thrombocytopenic purpura and neutropenia from ticlopidine must be weighed against potential benefits. In general, we recommend that patients continue to take aspirin through the day of surgery for all non–closed space, non–plastic surgical procedures. Other acute anticoagulant issues are addressed later (17,18).
TABLE 41.2 Concomitant Medical Therapy, Side Effects of Potential Concern Perioperatively, and Our Recommendations | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
There is substantial evidence that antihypertensive, plaque stress–reducing, lipid-lowering, and antiplatelet therapies decrease the stroke risk in the perioperative period as well as chronically. Our recommendations for management of concomitant medical therapy in the perioperative period are listed in Table 41.2.
Risk Assessment Algorithms
Although the foregoing disorders associated with vascular disease and complex surgery (diabetes, smoking and its sequelae, chronic pulmonary disease, hypertension, renal insufficiency, and ischemic heart disease) are the most common, it is their consequences that are most important to perioperative outcome. Understanding the end-organ effects of these diseases can guide appropriate perioperative therapy. It would be suboptimal to administer anesthesia to patients with uncontrolled medical conditions such as severe hypertension, a recent myocardial infarction, uncontrolled diabetes and hyperglycemia, or untreated pulmonary infections. However, an expanding aneurysm, crescendo transient ischemic attacks, or threatened limb loss can force one’s hand. In such situations, attempts to rapidly control chronically deranged blood pressure (which could precipitate cerebral ischemia) or electrolytes (which could, e.g., result in accidental administration of a bolus of potassium) may be more hazardous than leaving the condition untreated or trying to control the abnormality slowly. The National Veterans Affairs Surgical Risk Study found that low serum albumin values and high American Society of Anesthesiologists (ASA) physical classification were among the best predictors of morbidity and mortality after vascular surgery (19,20) (Table 41.3).
Whereas chronic medical conditions increase the likelihood of postoperative morbidity and mortality, postoperative complications have even greater predictive value for adverse outcomes (Table 41.4) (21). Other causes of morbidity after vascular and other complex surgery include bleeding, pulmonary infections, graft infections, renal insufficiency and failure, hepatic failure, cerebrovascular accidents, and spinal cord
ischemia resulting in paraplegia. The incidence of these other causes of morbidity has declined substantially in the last 20 years. Although multisystem organ failure may account for an increasing proportion of deaths after vascular surgery and complex vascular surgery, we believe that maintaining adequate cardiac function and perfusion of vital organs remains a vital aspect of reducing perioperative mortality. The factors that affect patient prognosis after vascular and complex surgery remain primarily related to the heart (1). We will therefore examine more closely the effects of known or suspected coronary artery disease on patient management before, during, and after vascular and other complex surgery.
ischemia resulting in paraplegia. The incidence of these other causes of morbidity has declined substantially in the last 20 years. Although multisystem organ failure may account for an increasing proportion of deaths after vascular surgery and complex vascular surgery, we believe that maintaining adequate cardiac function and perfusion of vital organs remains a vital aspect of reducing perioperative mortality. The factors that affect patient prognosis after vascular and complex surgery remain primarily related to the heart (1). We will therefore examine more closely the effects of known or suspected coronary artery disease on patient management before, during, and after vascular and other complex surgery.
TABLE 41.3 The 10 Most Important Preoperative Predictors of Postoperative 30-Day Mortality After Vascular Surgery in Veteran’s Affairs Medical Centers | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
TABLE 41.4 Demographics, Hospital Characteristics, Preoperative Chronic Medical Conditions, and Postoperative Complications and their Contributions to Mortality Prediction | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Coronary Artery Disease in Patients with Peripheral Vascular Disease
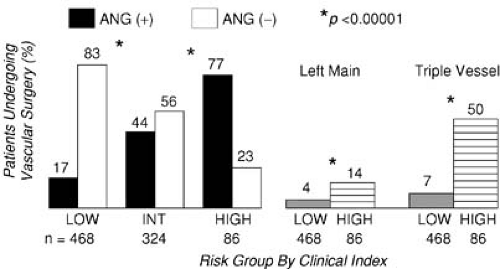
Hertzer et al. (22) performed coronary angiography in 1,001 consecutive patients presenting for vascular surgery and identified severe correctable coronary artery disease in 25% of the entire series. The incidence of significant coronary artery disease (stenosis >70%) detected by angiography was 78% in those with clinical indications of coronary artery disease and 37% in patients without any clinical indications. However, subsequent analysis demonstrated that clinical risk factors still predicted the severity of coronary artery disease (Fig. 41.1) (23). The absence of severe coronary stenoses can be predicted with a positive predictive value of 96% for patients who had none of the following risk factors: history of diabetes, prior angina, previous myocardial infarction, and congestive heart failure.
Short-term postoperative morbidity and mortality after vascular surgery is higher than after other types of complex noncardiac surgery (24). Long-term morbidity and mortality after vascular surgery are greatly influenced by the presence of coronary artery disease. The presence of uncorrected coronary artery disease appears to double 5-year mortality after vascular surgery. Coronary artery bypass graft (CABG) is associated with improved survival in peripheral vascular disease patients who have triple-vessel, but not single- or double-vessel, coronary artery disease (25). Previous percutaneous transluminal coronary angioplasty (PTCA) may protect against
perioperative cardiac events after vascular surgery. The prevalence of asymptomatic coronary artery disease and the substantial short-term and long-term cardiac morbidity and mortality in patients undergoing vascular surgery have led investigators and clinicians to propose and undertake extensive preoperative evaluation to detect underlying coronary artery disease.
perioperative cardiac events after vascular surgery. The prevalence of asymptomatic coronary artery disease and the substantial short-term and long-term cardiac morbidity and mortality in patients undergoing vascular surgery have led investigators and clinicians to propose and undertake extensive preoperative evaluation to detect underlying coronary artery disease.
Before discussing preoperative workup for detecting underlying coronary artery disease (CAD) it is important to review the pathophysiology that leads to perioperative MI or postoperative cardiovascular dysfunction. In addition to CAD, other important etiologic factors for perioperative cardiovascular events are perioperative hypothermia; significant valvular heart disease, especially aortic stenosis; history of congestive heart failure (CHF); anemia; increased intraoperative bleeding as assessed by the amount of cell-salvaged blood; increased circulating catecholamines; exogenous vasoconstrictors; and hypercoagulability (easily activated platelets with a higher fibrinogen rate and decreased fibrinolysis) (26,27,28,29




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree