Introduction
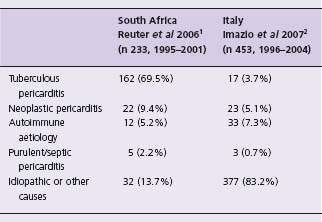
A wide variety of conditions may result in pericardial disease, which presents clinically as acute pericarditis, pericardial effusion or constrictive pericarditis. The etio-logic spectrum depends on the epidemiologic setting of the patient. For example, in industrialized countries, most cases of pericarditis are idiopathic, whereas tuberculosis accounts for the majority of patients in the developing world (Table 52.1).1,2 In many cases, pericardial disease is associated with a known condition (e.g. malignancy) or underlying cardiac disease, which prove to be the cause of pericardial disease. In patients with no apparent cause of the pericardial syndrome, the presence of inflammatory signs is predictive of acute pericarditis; on the other hand, a large effusion without inflammatory signs or tamponade is predictive of chronic idiopathic pericardial effusion.3 Tamponade without inflammatory signs is suspicious for neoplastic pericardial effusion. In patients living in tuberculosis-endemic regions, a pericardial effusion without apparent cause is likely to be tuberculous until proven otherwise. The prognosis of pericardial disease is related to the underlying disease, being especially poor in patients with malignancy.3
This overview deals with the clinical management of the common pericardial diseases, including idiopathic pericarditis, purulent pericarditis, tuberculous pericarditis, and neoplastic pericarditis. We examine the extent to which existing treatments are supported by evidence from well-designed prospective studies. The findings reported here are based on a comprehensive search of electronic databases and bibliographies of articles on pericarditis.
Epidemiology
Acute idiopathic pericarditis accounts for 80–90% of primary acute pericardial disease (i.e. pericardial syndrome without apparent cause at initial clinical evaluation) in Western countries.2,4,5 Incidence has been estimated at 28 per 100 000 per year, but the influence of the tested population, mode of presentation and seasonal and geographic variation of viral infections is significant.6 Acute pericarditis is responsible for ∼ 1% of presentations to emergency departments with electrocardiographic ST elevation, but at least an additional 10% of patients are diagnosed in the absence of electrocardiographic criteria.7,8
Etiology
The etiology of idiopathic pericarditis is presumed to be viral or autoimmune. However, in a French study of 136 patients diagnosed with idiopathic pericarditis after standard investigations and pericardiocentesis, serologic antibody evaluation and viral culture of throat swabs yielded alternative diagnoses in 39 (29%); the commonest causes were Coxiella burnetii (n = 10), enterovirus (n = 8), Mycoplasma infection (n = 4) and autoimmunity (high antinuclear antibody titer, n = 3).9 Previously unsuspected hypothyroidism was diagnosed in an additional 14 patients through routine measurement of thyroid-stimulating hormone. The use of more sophisticated methods for examining pericardial fluid and tissue, such as tumor markers, fluorescence-activated cell sorting, polymerase chain reaction and immunohistochemistry, as in the Marburg Pericarditis Registry, means that less than 5% of cases are labeled as idiopathic and a much higher proportion of cases are found to be viral or autoimmune.10
Diagnosis
Acute pericarditis is the occurrence of two or more of the following: characteristic chest pain, pericardial friction rub (pathognomonic of acute pericarditis), and an electrocardiogram showing characteristic ST segment elevation or typical serial changes. Transthoracic echocardiography is recommended for all patients.10,11 Cardiac tamponade is an indication for therapeutic pericardiocentesis. Diagnostic pericardiocentesis is recommended if an infective or malignant cause of effusion is suspected.10,11 Although there are no absolute clinical differentiators, a history of malignancy or autoimmune illness, high fever with rigors, skin rash or weight loss point to a specific disease. Blood tests may reveal renal failure while a markedly raised white cell count suggests purulent pericarditis.11
A recent prospective evaluation of 453 patients identified fever >38°C (odds ratio (OR) 3.56), subacute course (3.97), large effusion or tamponade (2.15), and failure of aspirin or NSAID therapy (2.50) as predictive of a specific cause being present (noted in 76 patients (17%)) with identified diagnoses of autoimmune, neoplastic, tuberculous or bacterial pericarditis.2 In another prospective study of 130 patients, the presence of inflammatory signs (characteristic chest pain, pericardial friction rub, fever or typical electro-cardiographic changes) in the absence of an identified cause of moderate to large effusion predicted the diagnosis of idiopathic pericarditis (OR 5.4), whereas when these were not present, tamponade predicted neoplasia (OR 2.9).12 This is in stark contrast to tuberculosis and HIV -endemic areas where the majority of effusions are caused by tuberculosis.13 The presence of features suggestive of a specific cause should prompt the clinician to investigate the patient further for an infective, neoplastic or autoimmune cause of the pericarditis.
Management
When none of the above features are present to suggest high risk and the presence of autoimmune, neoplastic, tuberculous or bacterial pericarditis, management in a day case unit has been shown to be safe as long as these low-risk patients are observed for a few hours while preliminary investigations are being undertaken and closely followed up thereafter to ensure response to treatment.8 Acute pericarditis can therefore be triaged based on clinical and echo-cardiographic criteria and those with high-risk features admitted and thoroughly investigated2 (Class I, Level C1).
It is generally accepted that bed rest and oral NSAIDs are effective in most patients with acute pericarditis, although there are no controlled trials.4,10,14 Ibuprofen has been recommended over indometacin due to its better side effect profile, larger dose range and anecdotal efficacy.10,14 Ketorolac is an extremely potent analgesic agent which has been reported to cause rapid resolution of symptomatic acute pericarditis in an uncontrolled study of 20 patients.15 However, before using expensive NSAIDs, it is appropriate to consider aspirin.16 In a prospective uncontrolled study of 254 patients with uncomplicated acute pericarditis, aspirin given at a dose of 800 mg every 6–8 hours for 7–10 days (in conjunction with misoprostol or omeprazole for gastric protection) with gradual tapering over 2–3 weeks resulted in a favorable response in 87%.8 Although similar in presentation to responders, those 33 of 254 patients with poor response to aspirin (defined as persistence of fever, a new pericardial effusion, or worsening of general illness after seven days) were shown to have a higher incidence of autoimmune (39% versus 2%) and tuberculous pericarditis (18% versus 0%). The differences in etiology of pericarditis between responders and nonresponders probably explain the reduced incidence of recurrent pericarditis (10% versus 61%) and constriction by one year in responders (0.5% versus 9%).8 In view of this evidence, response to a trial of aspirin or NSAID can be taken as predictive of good prognosis and no further investigation is routinely required. Conversely, failure of aspirin or NSAID therapy should prompt further investigation for a specific cause of pericarditis2,8 (Class I, Level C1).
A randomized open-label trial of COlchicine for acute PEricarditis (COPE study) in 120 patients with pericarditis due to idiopathic, viral, postpericardiectomy and connective tissue disease demonstrated on intention-to-treat analysis that a three-month course of colchicine in addition to a 3–4 week course of aspirin reduced symptom persistence at 72 hours (from 37% to 12%, P = 0.003) and recurrence rate at 18 months (32% to 11%, P = 0.004, number needed to treat (NNT) = 5).17 No serious adverse effects of colchicine were seen but the drug had to be discontinued in 8% of patients due to diarrhea. The results of this trial will be supplemented with a larger trial of colchicine versus placebo currently underway.18 Colchicine (0.5mg bd)10 added to an NSAID/aspirin (Class I, Level B) or as monotherapy (Class IIa, Level C2) appears to be effective for the initial attack and prevention of recurrences of acute pericarditis.
A preliminary study has highlighted the potential ben-efits of rosuvastatin as adjunctive therapy to NSAIDs in acute pericarditis.19 The rationale for the use of statins in this setting is based on their known anti-inflammatory effects, as shown by the lowering of C-reactive protein (CRP) independently of their lipid-lowering effect.19 Fifty five consecutive patients with a first episode of acute pericarditis were randomized to either indometacin plus placebo or indometacin plus rosuvastatin 10 mg, with treatment continued for a week after inflammatory markers normalized. The addition of rosuvastatin resulted in sig-nificantly earlier normalization of CRP levels (5.0 versus 6.0 days, P = 0.022), ST segment deviation (3.5 vs 4.5 days, P = 0.001), pericardial effusion (4.5 vs 5.5 days, P = 0.001) and erythrocyte sedimentation rate (ESR) (5.0 vs 6.0 days, P = 0.022). These promising results need to be confirmed in larger studies before application to clinical practice (Class IIb, Level B).
Corticosteroids are effective in acute pericarditis but observational data suggest that their use in this setting is associated with an increased risk of recurrence.16,20 The pain and any associated fever, leukocytosis, and other inflam-matory factors resolve rapidly with high-dose corticoste-roid administration (e.g. 1–1.5 mg/kg/day), only to return during tapering to a low dose of the steroid.16 Prospective data from the COPE trial add to these observations.17 Patients in both arms of the study with aspirin intolerance or contraindication (n = 19, 16%) were treated with corti-costeroids for a similar duration to aspirin. Steroid use was an independent risk factor for recurrence on multivariate analysis (OR 4.30, 95% confidence interval (CI) 1.21–15.25). Therefore corticosteroids are restricted to cases of autoimmune or connective tissue disease with early introduction of NSAIDs or colchicine during the tapering phase (Class IIa, Level C2).10 Intrapericardial triamcinolone is one way of reducing systemic side effects which have been shown to be effective in up to 93% of patients with autoimmune pericardial effusion21 (Class I, Level C2).
Recurrent pericarditis
Recurrent pericarditis is the most troublesome complication of acute pericarditis. The criteria for the diagnosis of recurrent pericarditis are a documented first attack of acute pericarditis, and evidence of either recurrence or continued activity of pericarditis despite therapy.22 Recurrent pericarditis, which affects about 20% of cases,20,22 is diagnosed in the presence of chest pain and one or more of the following: pericardial friction rub, electrocardiographic changes, echocardiographic evidence of pericardial effusion, and raised inflammatory markers.22,23 Approximately 40% have multiple attacks and 10% have more than five attacks over several years, although attacks tend to become progressively less severe.20 The disease process can continue for up to 43 years (mean 5.4 years), with symptom-free periods ranging from one month to 39 years (median three months), but in most it gradually “burns out” after 10 years.24 Despite this, prognosis is good: cardiac tamponade and pericardial constriction are very rare, myocardial disease is not seen, and atrial fibrillation is an uncommon transient feature of exacerbations.23–25
There is no single consistent etiologic factor but evidence of ongoing viral infection, prior corticosteroid use, autoimmunity and familial clustering have all been variably reported.16,20 Antinuclear antibodies have been reported in approximately 55% and a new diagnosis of connective tissue disease in 10% of 61 patients with recurrent idiopathic pericarditis.24 Where patients from Mediterranean countries are over-represented, familial Mediterranean fever has been reported in 4%.26
In the assessment of patients with recurrence, evaluation for connective tissue disease is appropriate but complex diagnostic procedures, such as invasive endomyocardial or pericardial sampling, are usually not justified.16,20
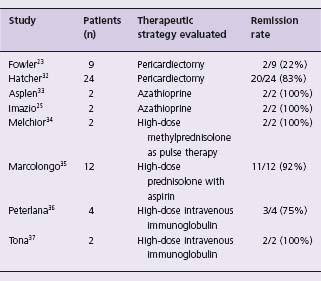
The open-label COlchicine for REcurrent pericarditis (CORE) study randomized 84 consecutive patients with first recurrence of idiopathic, autoimmune or viral pericarditis to aspirin or aspirin and a six-month course of colchicine.22 On intention-to-treat analysis, treatment with colchicine significantly decreased 18-month recurrence from 51% to 24% (P = 0.02, NNT 4) and symptom persistence at 72 hours from 31% to 10% (P = 0.03).10 Colchicine (2 mg/day for one or two days, followed by 1 mg/day)10 can be recommended as effective therapy for recurrent pericarditis (Class I, Level B). Prednisolone was substituted in 30 patients with aspirin contraindication and on multivariate analysis this was an independent risk factor for further recurrences (OR 2.9, 95% CI 1.1–8.3). These find-ings confirm uncontrolled studies reporting on the use of colchicine as first-line therapy for recurrence25,27,28 and the deleterious effects of corticosteroids in attenuating its effectiveness26 and increasing the risk of further recurrence.20,25 A sustained response on discontinuation of colchicine is seen in 60%, although recurrences are minor and resolve with reinstitution of colchicine (Class IIa, Level C2).28 Relapse on colchicine can be managed effectively in the majority with reinstitution of NSAIDs.28 Patients with recurrent pain despite NSAIDs and colchicine are challenging yet there is a paucity of evidence to guide management. Corticosteroids are recommended in European guidelines only in patients with poor general condition or frequent crises 10 (Class IIa, Level C2). An extended course of low -dose steroids may be superior to regimes utilizing a high dose initially. A nonrandomized retrospective comparison reported a significantly increased risk of recurrence with a higher dose: 33 of 51 patients taking prednisone 1.0 mg/ kg/day suffered a recurrence by mean followup of 58 months as compared to 16 of 49 taking 0.2–0.5 mg/kg/day by 54 months (hazards ratio 3.6, P < 0.001). Some of this may be related to premature withdrawal of therapy as the higher dose was associated with significantly increased toxicity (osteoporosis and Cushing’ s syndrome reported in 24% vs 2%).28a (Class IIa, Level C1) Claims of effectiveness have been made in small uncontrolled studies for pericardiectomy, azathioprine, chloroquine, and intravenous immunoglobulin (Table 52.2) (Class IIb, Level C2).
Table 52.2 Therapeutic strategies previously evaluated in recurrent pericarditis (after failure of NSAID)
Chronic idiopathic pericardial effusion
Patients with large pericardial effusion of no apparent cause which persists for more than three months constitute 3% of pericarditis and 70% of large chronic effusions in the West.29 The main concern is the occurrence of tamponade, reported in 30–40% of cases, at times with unexpected decompensation after being asymptomatic for several years.29,30 For this reason, drainage is recommended even in the absence of tamponade (Class IIa, Level C1). Lasting remission is seen in 33–75% after therapeutic pericardio-centesis alone and 95% after extended catheter drainage.30 Pericardiectomy offers long-term success whether carried out initially or after failure of percutaneous drainage.29–31 Survival rates are similar to the background population30 but a late appearance of malignancy has been reported in 5–10% of cases at 6–80 months following diagnosis.29,30
Epidemiology
Purulent pericarditis is now rare in the developed world, comprising 0.7–5% of pericardial effusions2,12,38,39 and less than 1% of cases undergoing pericardiectomy.40–42 In Western series, the most common reported organisms have been staphylococci, streptococci and pneumococci when the predominant associated lesions are empyema (50%) or pneumonia (33%).43 In a series where over 50% of patients had compromised immunity or thoracic surgery, Staphylococcus aureus (30%) and fungi (20%) have predominated.44 In another unselected retrospective series anaerobes originating from the oropharynx were most commonly found.45 Seeding may be hematogenous but contiguous spread from less common sites such as the retropharyngeal space, cardiac valves, and below the diaphragm must be excluded.46 Neisseria meningitidis may involve the pericardium in two ways: either through initiating an immune-mediated sterile effusion47,48 or by direct infection and purulent reaction.49 The modern era of iatrogenic and HIV-associated immunosuppression has witnessed more unusual organisms and presentations.50–54
Diagnosis
A prodrome of 3–10 days precedes presentation with fever, cough, chest pain and tachycardia.43,44 Pericardial rub is present in 35–45% of patients and tamponade in 40–80%. However, when pericardial involvement is secondary, the underlying sepsis predominates the illness and its outcome.55 This may partly explain why as many as 30–40% of patients are diagnosed post mortem43,44 and also stresses the importance of identifying associated foci, with the use of adjunctive investigations as indicated.
Blood tests are in keeping with sepsis whilst radiographic cardiomegaly (75%), lung field shadowing (50%) and pleural effusions (30%) are common. Electrocardiography is usually typical of myopericarditis but may be normal in 10–30% of patients. Echocardiography confirms the presence of effusion; however, in patients with sepsis this is not proof in itself of pericardial infection.56 Suspicion of purulent pericarditis is an indication for urgent pericardiocentesis,10 which is diagnostic.57 The fluid may be frankly purulent. A low pericardial to serum glucose ratio (mean 0.3) and raised pericardial fluid white cell count with high proportion of neutrophils (mean cell count 2.8 per μl, 92% neutrophils) differentiate from tuberculous (ratio 0.7, count 1.7 per μl, 50% neutrophils) and neoplastic (ratio 0.8, count 3.3 per μl, 55% neutrophils) pericarditis.58 Fluid should be sent for bacterial, fungal and tuberculous studies, blood cultures drawn and other samples taken as guided by the clinical presentation.
Management
Purulent pericarditis should be managed aggressively as mortality is universal if untreated, whereas with comprehensive therapy 85% have been reported to survive the episode and have good long -term outcome.43 Intravenous antimicrobial therapy should be started empirically until microbiologic results are available. Effective pericardial drainage is crucial.10 There are no prospective studies comparing one modality over another in purulent pericarditis. Retrospective comparisons with unselected cases feature purulent pericarditis rarely and in small numbers. Central to the choice of modality is the fact that purulent effusions are often heavily loculated and likely to reaccumulate rapidly. Subxiphoid pericardiostomy and rinsing of the pericardial cavity is recommended.10 This allows more complete drainage of the effusion as loculations can be manually lyzed at the time of surgery. In a Zimbabwean report of 21 cases of purulent pericarditis managed as such, under local anesthesia in most cases, there was one recurrence of effusion (5%), one case of constriction (5%), and four deaths (20%)59 (Class IIa, Level C2).
Intrapericardial thrombolysis through a percutaneous catheter has attempted to address the problems encountered with loculations restricting adequate drainage. Ten studies have reported a total of 35 patients with purulent pericardial effusion treated with percutaneous intrapericardial thrombolysis.57,60–68 Intrapericardial thrombolysis did not alter systemic clotting parameters and was associated with regression or disappearance of intrapericardial fibrin strands, improvement in hemodynamic status and prevention of constriction. Treatment failure was reported in two patients. Reported complications were intrapericardial haemorrhage (n = 2) and mortality from ongoing sepsis (n = 1). A controlled study of intrapericardial urokinase in 60 patients with tuberculous pericarditis and 34 with purulent pericarditis reported a 13% incidence of intrapericardial haemorrhage.69 An adequately designed study is required to evaluate the safety and effectiveness of this promising therapy in selected patients with purulent effusion and subacute constriction (Class IIb, Level C2). Pericardiectomy is recommended for dense adhesions, loculated or thick purulent effusion, recurrence of tampon-ade, persistent infection, and progression to constriction, with up to 8% surgical mortality (Class IIa, Level C2).10 Duration of antimicrobial therapy has to be individualized (Class IIa, Level C2).
Epidemiology
Tuberculous pericarditis accounts for less than 5% of pericardial disease in the developed world.2,4,5 By contrast, tuberculosis is the cause of large pericardial effusions in over 90% of HIV-infected70–72 and 50–70% of non-HIV infected individuals who live in tuberculosis-endemic regions and communities.72,70 The disease can occur at any age73,74 and men are more frequently affected than women, though occasionally a greater proportion of female patients has been reported.75–78 Clinical presentations are as pericar-dial effusion (80% of cases), effusive-constrictive pericarditis (15%) or constrictive pericarditis (5%).79 Tuberculous pericarditis is a serious condition with a mortality of 17–40%; constriction occurs in a similar proportion of cases following tuberculous pericardial effusion.80
Tuberculous pericardial effusion
Tuberculous pericardial effusion should be suspected in all instances of pericarditis without a rapidly self-limiting course. Onset is insidious and presentation is with nonspecific systemic symptoms, such as fever, night sweats, fatigue, and weight loss.13 Chest pain, cough, and breathlessness are common, although severe pericardial pain of acute onset characteristic of idiopathic pericarditis is unusual. Right upper abdominal aching owing to liver congestion is also common. In African patients with tuberculous pericardial effusions, evidence of chronic cardiac compression mimicking heart failure is the most common presentation and 10% present with clinical tamponade.75 While there is marked overlap between the physical signs of pericardial effusion and constrictive pericarditis, the presence of a pericardial friction rub and increased cardiac dullness extending to the right of the sternum favor a clinical diagnosis of pericardial effusion.81
Diagnosis
The chest radiograph, which shows an enlarged cardiac shadow in almost all cases,82 demonstrates features of active pulmonary tuberculosis in 30% of cases and pleural effusion in 40–60% of cases.75,82–85 The electrocardiogram is abnormal in virtually all cases, usually in the form of nonspecific ST T-wave changes.86 The PR segment deviation and ST segment elevation characteristic of acute pericarditis are found in only 9–11% of cases. Atrial fibrillation is usually transient. Echocardiographic findings of effusion with fibrinous strands on the visceral pericardium are typical but not specific for a tuberculous aetiology87,88 Imaging by CT scanning or MRI can also be used to provide incremental information to echocardiography89–91 In addition to features of pericardial disease (i.e. pericardial effusion and thickening of the pericardium), CT of the chest shows typical changes in mediastinal lymph nodes (i.e. enlargement >10 mm with matting and hypodense centers and sparing of hilar lymph nodes) in almost all cases which resolve on treatment.89 MRI has the added advantage of assessing the extent of pericardial inflammation91,92 and myocardial involvement.93,94
A “definite or proven” diagnosis of tuberculous pericarditis is based on the demonstration of tubercle bacilli in pericardial fluid or on histologic section of the pericardium, and a “probable or presumptive” diagnosis is made when there is proof of tuberculosis elsewhere in a patient with unexplained pericarditis, a lymphocytic pericardial exudate with elevated adenine deaminase (ADA), interferon-gamma (IFN-γ) or lysozyme levels, and/or an appropriate response to antituberculosis chemotherapy13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree