Anatomy of the Pericardium
The pericardium is composed of visceral and parietal components. The visceral pericardium is a mesothelial monolayer that adheres firmly to the epicardium, reflects over the origin of the great vessels, and—together with a tough, fibrous coat—envelops the heart as the parietal pericardium (Fig. 85–1). The pericardial space is enclosed between these two serosal layers and normally contains up to 50 mL of a plasma ultrafiltrate, the pericardial fluid. Pericardial reflections around the great vessels tether the pericardium superiorly and result in the formation of two potential spaces: the oblique and transverse sinuses. Superior and inferior pericardiosternal and diaphragmatic ligaments limit displacement of the pericardium and its contents within the chest and neutralize the effects of respiration and change of body position. The phrenic nerves are embedded in the parietal pericardium and, for this reason, are vulnerable to injury during pericardial resection.
Figure 85–1.
Computed tomography scan shows the normal pericardium as a thin, curvilinear line (open arrows). The increased thickening over the anterior surface of the heart (solid arrows) is probably an artifact from transmitted right ventricular (RV) pulsations. Reproduced with permission from Moncada R, Baker M. In: Higgins CB, ed. CT of the Heart and Great Vessels. Mt. Kisco, NY: Futura; 1983:292.
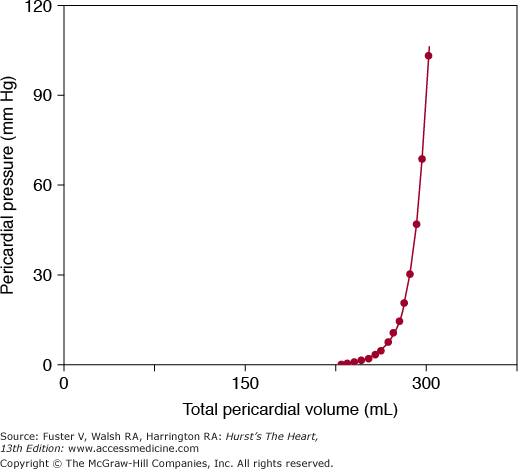
Histologically, the pericardium is composed predominantly of compact collagen layers interspersed with elastin fibers. The abundance and orientation of the collagen fibers are responsible for the characteristic viscoelastic mechanical properties of the pericardium. For example, the pressure-volume relation of the pericardium is nonlinear; that is, the relation is initially flat (producing little to no change in pressure for large changes in volume) and develops a “bend” or “knee” at a critical pressure, which terminates in a steep slope (producing large changes in pressure for small changes in volume) (Fig. 85–2). In addition, the pericardium is anisotropic; that is, it stretches more in the short axis than in the long axis.
Physiology of the Pericardium
The pericardium is not essential for life; no adverse consequences follow congenital absence or surgical removal of the pericardium. However, the pericardium serves many important (although subtle) functions (Table 85–1). It limits distension of the cardiac chambers and facilitates interaction and coupling of the ventricles and atria.1 Thus, changes in pressure and volume on one side of the heart can influence pressure and volume on the other side. Limitation of cardiac filling volumes by the pericardium may also limit cardiac output and oxygen delivery during exercise.1 The pericardium also influences quantitative and qualitative aspects of ventricular filling; the thin-walled right ventricle (RV) and atrium are more subject to the influence of the pericardium than is the more resistant, thick-walled left ventricle (LV).
| Mechanical |
| Effects on chambers |
| Limits short-term cardiac distention |
| Facilitates cardiac chamber coupling and interaction |
| Maintains pressure-volume relation of the cardiac chambers and output from them |
| Maintains geometry of left ventricle |
| Effects on whole heart |
| Lubricates, minimizes friction |
| Equalizes gravitation and inertial hydrostatic forces |
| Mechanical barrier to infection |
| Immunologic |
| Vasomotor |
| Fibrinolytic |
| Modulation of myocyte structure and function and gene expression |
| Vehicle for drug delivery and gene therapy |
Although the magnitude and importance of pericardial restraint of ventricular filling at physiologic cardiac volumes remain controversial, there is general agreement that pericardial reserve volume (ie, the difference between unstressed pericardial volume and cardiac volume) is relatively small and that pericardial influences become significant when the reserve volume is exceeded. This may occur with rapid increases in blood volume and in disease states characterized by rapid increases in heart size (eg, acute mitral and tricuspid regurgitation, pulmonary embolism, RV infarction). In contrast, chronic stretching of the pericardium results in “stress relaxation”; this explains why large but slowly developing effusions do not produce tamponade. In addition, the pericardium adapts to cardiac growth by “creep” (ie, an increase in volume with constant stretch) and cellular hypertrophy.
The pericardium serves a variety of other important functions. It prevents excessive torsion and displacement of the heart, minimizes friction with surrounding structures, and is an anatomic barrier to the spread of infection from contiguous structures. The thin layer of pericardial fluid reduces friction on the epicardium and equalizes gravitational, hydrostatic, and inertial forces over the surface of the heart; therefore, transmural cardiac pressures do not change during acceleration or differ regionally within cardiac chambers. The pericardium also has immunologic, vasomotor, paracrine, and fibrinolytic activities.2 The mesothelium of the pericardium is metabolically active and produces prostaglandin E2, eicosanoids, and prostacyclin; these substances modulate sympathetic neurotransmission and myocardial contractility and may influence epicardial coronary arterial tone. Epicardial mesothelial cells may modulate myocyte structure and function and gene expression. The level of brain natriuretic peptide (BNP) in the pericardial fluid is a more sensitive and accurate indicator of ventricular volume and pressure than is either plasma BNP or atrial natriuretic factor; it may play an autocrine-paracrine role in heart failure. Finally, the pericardial space has been used as a vehicle for drug delivery and gene therapy; studies using radiolabeled growth factors indicate that substances more consistently and reproducibly gain access to the coronary arteries via pericardial fluid than via endoluminal delivery.3
Pathology of the Pericardium
In view of the pericardium’s simple structure, clinicopathologic processes involving it are understandably few; indeed, pericardial heart disease includes only pericarditis (an acute, subacute, or chronic fibrinous, “noneffusive,” or exudative process) and its complications, tamponade and constriction (an acute, subacute, or chronic adhesive, fibrocalcific response), and congenital lesions. However, despite a limited number of clinical syndromes, the pericardium is affected by virtually every category of disease, including infectious, neoplastic, immune-inflammatory, metabolic, iatrogenic, traumatic, and congenital etiologies. Thus, the physician is likely to encounter patients with pericardial disease in a variety of settings, either as an isolated phenomenon or as a complication of a variety of systemic disorders, trauma, or certain drugs. In these settings, pericardial involvement may be overshadowed by extracardiac manifestations and difficult to recognize.
Treatment of pericardial disease is also challenging in that there is a paucity of randomized, placebo-controlled trials (Level of Evidence: A) from which appropriate therapy may be selected and important clinical decisions assisted. Table 85–2 summarizes European Society of Cardiology guidelines.4
| Indication | Evidence | |
|---|---|---|
| Acute pericarditis | ||
| NSAIDs | Class I | Level B |
| Colchicinea | Class IIa | Level B |
| Systemic corticosteroidsb | Class IIa | Level B |
| Chronic pericarditis | ||
| Balloon pericardiotomy or pericardiectomyc | Class IIb | Level B |
| Recurrent pericarditis | ||
| Colchicine | Class I | Level B |
| Systemic corticosteroidsd | Class IIa | Level C |
| Pericardiectomye | Class IIa | Level B |
| Pericardial effusion | ||
| Pericardiocentesis for cardiac tamponade | Class I | Level B |
| Pericardiocentesis for smaller effusions | Class IIa | Level B |
| Analysis of pericardial fluid | ||
| Pericardial fluid and blood for bacteria | Class I | Level B |
| PCR, ADA, IFγ, lysozyme for tuberculosis | Class I | Level B |
| PCR, in situ hybridization for virus | Class IIa | Level B |
| Serum viral titers | Class IIb | Level B |
| Pericardial chemistry (specific gravity, protein, LDH, glucose) | Class IIb | Level B |
| Specific forms of pericarditis | ||
| Corticosteroids for TB pericarditis | Class IIb | Level A |
| Pericardiocentesis for tamponade and large effusions unresponsive to dialysis | Class IIa | Level B |
| Pericardiocentesis for large neoplastic effusions | Class I | Level B |
| Diagnostic pericardiocentesis in suspected neoplastic effusion | Class IIa | Level B |
| Intrapericardial instillation of cytotoxic/sclerosing agent for neoplastic pericarditis | Class IIa | Level B |
| Radiation therapy for control of effusions in patients with radiosensitive tumors | Class IIa | Level B |
| Percutaneous balloon pericardiotomy for malignant effusions | Class IIa | Level B |
| Pleuropericardiotomy to drain malignant effusions | Class IIb | Level C |
| Surgical therapy of chylous effusion resistant to diet and pericardiocentesis | Class I | Level B |
| Thyroid hormone for effusion secondary to myxedema | Class I | Level B |
Acute Pericarditis
Acute fibrinous or dry pericarditis is a syndrome characterized by typical chest pain, a pathognomonic pericardial friction rub, and specific electrocardiogram (ECG) changes. Table 85–3 lists many of the conditions associated with acute pericarditis. The following description refers to viral and idiopathic pericarditis without significant effusion. Specific forms of pericardial heart disease are reviewed later in this chapter.
| Idiopathic |
| Infectious |
| Bacterial (Pneumococcus, Streptococcus, Staphylococcus, Haemophilus influenzae, gram-negative rods, Brucella melitensis, Francisella tularensis, Legionella pneumophila, Neisseria gonorrhoeae, Neisseria meningitidis, Borrelia burgdorferi [Lyme disease], Mycoplasma) |
| Viral (coxsackievirus, echovirus, adenovirus, varicella, influenza, cytomegalovirus, human immunodeficiency virus, hepatitis B, mumps, infectious mononucleosis) |
| Mycobacterial (Mycobacterium tuberculosis, Mycobacterium avium-intracellulare) |
| Fungal (Histoplasma, coccidioidomycosis, Blastomyces, Candida albicans, Nocardia, Actinomyces) |
| Protozoal (Toxoplasma, Echinococcus, amoebae) |
| Acquired immunodeficiency syndrome–associated |
| Neoplastic |
| Primary (mesothelioma, fibrosarcoma) |
| Secondary (breast, lung, melanoma, lymphoma, leukemia) |
| Immune/inflammatory |
| Connective tissue diseases (rheumatoid arthritis, systemic lupus erythematosus, scleroderma, acute rheumatic fever, dermatomyositis, mixed connective tissue disease, Wegener granulomatosis) |
| Arteritis (temporal arteritis, polyarteritis nodosa, Takayasu arteritis) |
| Acute myocardial infarction and post-myocardial infarction (Dressler syndrome) |
| Postpericardiotomy |
| Posttraumatic |
| Metabolic |
| Nephrogenic |
| Aortic dissection |
| Myxedema |
| Amyloidosis |
| Iatrogenic |
| Radiation injury |
| Instrument/device trauma (implantable defibrillators, pacemakers, catheters) |
| Drugs (hydralazine, procainamide, daunorubicin, isoniazid, anticoagulants, cyclosporine, methysergide, phenytoin, dantrolene, mesalazine) |
| Cardiac resuscitation |
| Traumatic |
| Blunt trauma |
| Penetrating trauma |
| Surgical trauma |
| Congenital |
| Pericardial cysts |
| Congenital absence of pericardium |
| Mulibrey nanism |
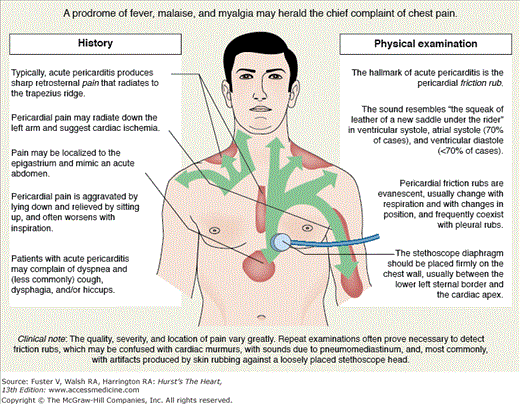
Acute pericarditis typically produces sharp retrosternal pain that radiates to the trapezius ridge and is aggravated by lying down and relieved by sitting up; its onset is frequently heralded by a prodrome of fever, malaise, and myalgia (Fig. 85–3). The pain of pericarditis is often worse with inspiration and is difficult to distinguish from pleurisy; in some cases, the pain is indistinguishable from that of myocardial infarction (MI). The quality, severity, and location of pain vary greatly, and chest pain may be absent in acute pericarditis, especially in early pericarditis complicating MI or cardiac surgery and in uremic pericarditis.
The hallmark of acute pericarditis is the pericardial friction rub; because of its superficial, creaky, or scratchy character, it often is likened to the sound of walking on dry snow or the squeak of a leather saddle (see Fig. 85–3). Rubs are heard anywhere over the precordium but most often between the lower left sternal edge and the cardiac apex; they are usually heard best with the diaphragm of the stethoscope applied firmly and with respiration suspended. Most pericardial friction rubs are independent of the respiratory cycle, but on occasion, they are louder during inspiration. The pericardial rub may be confined to ventricular systole but most often includes a component during atrial systole and occasionally during ventricular diastolic filling, resulting in biphasic and triphasic rubs, respectively. Biphasic rubs must be distinguished from murmurs of mixed aortic valve disease, and monophasic rubs are often mistaken for systolic murmurs. Frequent examinations are necessary to detect a rub because of its evanescent nature; pericardial fluid does not prevent a friction rub.
In uncomplicated pericarditis, the jugular venous pressure usually remains normal. Ventricular third and fourth heart sounds indicate coexisting myocardial disease. The history and physical examination are also helpful in recognizing complications and in identifying underlying diseases associated with pericarditis. Depending on the etiology, there may be fever and other signs of inflammation or systemic illness.
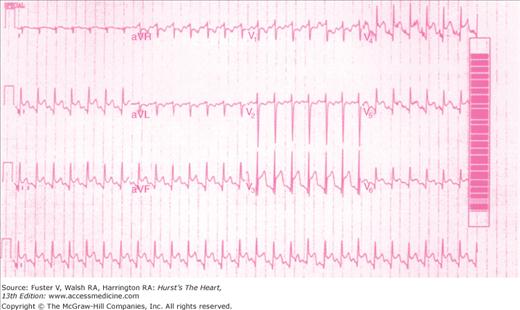
The ECG may either confirm the clinical suspicion of pericardial disease or first alert the clinician to the presence of pericarditis (Fig. 85–4). Serial tracings may be needed to distinguish the ST-segment elevations caused by acute pericarditis from those caused by acute MI or normal early repolarization. The ST-T wave changes in acute pericarditis are diffuse and have characteristic evolutionary changes. In the first stage, ST-segment elevations (which differ from ischemic ST-segment elevations by their upward concavity and seldom exceed 5 mm in height) typically occur within a few hours of the onset of chest pain and persist for hours or days. Depression of the PR segment (except in lead aVR) occurs in this stage and differentiates acute pericarditis from early repolarization variants. In the second stage, the ST segments return to baseline; at this point, the T waves may appear normal or exhibit a loss of amplitude. In the third stage, tracings show inversion of T waves. T wave inversions may persist indefinitely, particularly with tuberculous, uremic, or neoplastic pericarditis. The ECG normalizes in the variably present fourth stage. In a typical case of acute pericarditis, the approximate time frame for these ECG changes is 2 weeks. However, only approximately 50% of patients with acute pericarditis display all four ECG stages, and variations are common. Atrial arrhythmias complicate 5% to 10% of cases of acute pericarditis.
The ST-segment elevation seen in acute pericarditis can usually be distinguished from that of acute MI by the absence of Q waves, the upwardly concave ST segments, and the absence of associated T wave inversions. The acute ST-segment elevation of the Prinzmetal variant of angina is more transitory and is associated with ischemic pain. Although the ST-segment elevation in the early repolarization variant (common in young individuals, especially blacks, athletes, and psychiatric patients) may simulate the ECG of acute pericarditis, the former is distinguished by the absence of PR-segment depression and evolutionary ST-T wave changes.
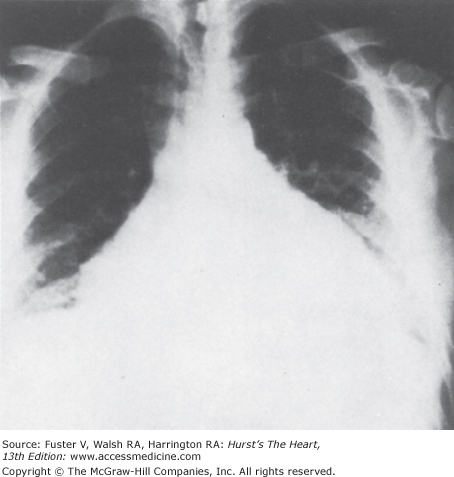
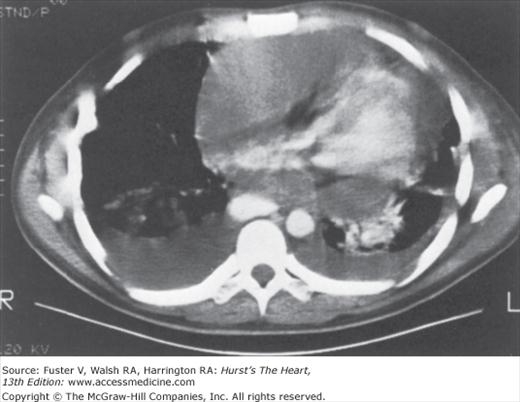
In uncomplicated acute pericarditis, the chest radiograph is generally normal. However, an enlarged cardiac silhouette may be evident because of a moderate or large pericardial effusion (Fig. 85–5). The chest radiograph may provide evidence of tuberculosis, fungal disease, pneumonia, or neoplasm.
The use of echocardiography for the evaluation of all patients with suspected pericardial disease was given a class I recommendation by a 2003 task force of the American College of Cardiology (ACC), the American Heart Association (AHA), and the American Society of Echocardiography (ASE).5 Echocardiographic identification of pericardial effusion confirms the clinical diagnosis of acute pericarditis (Fig. 85–6), but a patient with purely fibrinous acute pericarditis often has a normal echocardiogram. Echocardiography estimates the volume of pericardial fluid, identifies cardiac tamponade, suggests the basis of pericarditis, and documents associated acute myocarditis with congestive heart failure.
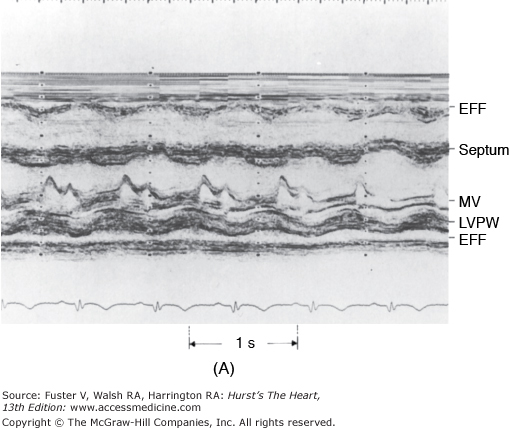
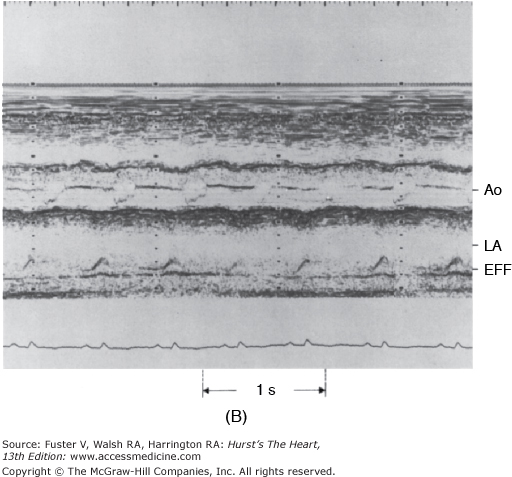
Figure 85–6.
M-mode echocardiograms of pericardial effusion (EFF). A. The effusion appears as an echo-free space posterior to the left ventricular posterior wall (LVPW). Note that parietal pericardium has relatively flat motion throughout the cardiac cycle. MV, mitral valve. B. Pericardial effusion behind the left atrium (LA). Note the exaggerated motion of the posterior left atrial wall. Ao, aorta. Reproduced with permission from Hoit BD. Imaging the pericardium. Cardiol Clin. 1990;8:588.
Nonspecific blood markers of inflammation, such as the erythrocyte sedimentation rate and the white blood cell count, usually increase in cases of acute pericarditis. Patients with extensive epicarditis occasionally have increases in serum cardiac isoenzymes suggestive of acute MI. In one series, nearly half of all patients presenting with acute, idiopathic pericarditis had increased serum troponin I levels, half of which were within the range considered diagnostic for acute MI.6 Although no significant coronary artery disease was detected in any of the patients who ultimately underwent coronary angiography, acute MI should remain high on the differential diagnosis in this setting because more than half of the patients with elevated troponin I presented with concurrent ST-segment elevation on ECG.
Hospitalization is warranted for many patients who present with an initial episode of acute pericarditis (particularly those with moderate or large effusions, myopericarditis, or high-risk features such as elevated temperature, subacute onset, immunosuppression, recent trauma, oral anticoagulant therapy, and aspirin or nonsteroidal anti-inflammatory drug [NSAID] failure)7 to determine the etiology and observe for cardiac tamponade; close follow-up is important in the remainder of patients. Establishing the exact cause of acute pericarditis is an important aspect of management, but considerable judgment must be exercised in deciding whether and how to investigate the possibility of concomitant systemic disease.
An extensive evaluation is generally unnecessary in a young, previously healthy adult who presents with a viral syndrome, typical pericardial chest pain, and a pericardial friction rub. Despite the availability of polymerase chain reaction (PCR) and histochemistry for etiopathogenetic classification, most cases of viral pericarditis are recognized long after the period of viral activity, making a specific etiologic diagnosis and the need for antiviral chemotherapy unnecessary. Depending on the history and symptoms at presentation, trauma, myocarditis, systemic lupus erythematosus (SLE), and/or purulent pericarditis require consideration in younger patients. In older adults, MI, tuberculosis, and especially neoplastic disease should be considered.
Acute pericarditis usually responds to oral NSAIDs (eg, aspirin [acetylsalicylic acid (ASA)] 650 mg every 3-4 hours or ibuprofen 300-800 mg every 6 hours). Prophylaxis against gastrointestinal bleeding with histamine-2 antagonists or proton pump inhibitors is warranted, particularly in those at high risk or who require longer durations of treatment. Cumulative anecdotal data, expert consensus, and an open-label clinical trial8 suggest that colchicine (1 mg/d, with or without a 2-mg loading dose for 3 months), either as a supplement to the use of NSAIDs or as monotherapy, is effective for the acute episode, is well tolerated, and may prevent recurrences; accordingly, it has been given a class IIa recommendation in the European guidelines. Adverse effects of colchicine (eg, diarrhea, nausea, abdominal pain) are usually mild and most often do not necessitate withdrawal of the drug; common adverse effects (1%-10%) at higher doses include elevated transaminases and alopecia. Bone marrow suppression, hepatotoxicity, and myotoxicity are less common, and azoospermia is rare. Colchicine is contraindicated in those with a hypersensitivity to colchicine; severe renal, gastrointestinal, hepatic, or cardiac disorders; blood dyscrasias; and pregnancy.9
Chest pain is usually alleviated in 1 to 2 days, and the friction rub and ST-segment elevation resolve shortly thereafter. Most mild cases of idiopathic and viral pericarditis are adequately treated with 1 to 4 days of treatment; however, the duration of therapy is variable, and patients should be treated until an effusion, if present, has resolved. The intensity of therapy is dictated by the distress of the patient, and narcotics may be required for severe pain. Some cases necessitate steroid therapy (prednisone 60-80 mg/d) for a week to control pain, with the dosage tapered carefully on an individual basis thereafter. However, corticosteroids should be avoided unless there is a specific indication (such as connective tissue disease or autoreactive or uremic pericarditis) because they enhance viral multiplication and may result in recurrences when the dosage is tapered; colchicine may be a particularly useful adjunct in this situation. Importantly, tuberculous and pyogenic pericarditis should be excluded before steroid therapy is initiated. Intrapericardial instillation of triamcinolone (300 mg/m2) avoids systemic adverse effects and is highly effective.10 Patients in whom pericarditis represents one manifestation of systemic illness (such as sepsis, uremia, connective tissue disease, or neoplasia) should, in addition to palliative and supportive treatment, also receive therapy directed toward the primary disorder. Recurrences are reported to occur in 20% to 50% of patients.11
Recurrent Pericarditis
Recurrent or relapsing acute pericarditis is one of the most distressing disorders of the pericardium for both patient and physician; it may occur with or without pericardial effusion and is occasionally associated with pleural effusion or parenchymal pulmonary lesions. Recurrences occur with highly variable frequency over a course of many years. The reasons for recurrence are unclear, but the phenomenon suggests that acute pericarditis itself may represent or generate an autoimmune process; reactivation of viral pericarditis, an unrelated infection, or corticosteroid use may be responsible. Recurrences may be spontaneous (ie, occurring at varying intervals after discontinuation of drug) but more commonly are “incessant,” associated with discontinuation or tapering doses of anti-inflammatory drugs. When associated with pericardial effusion, recurrent pericarditis can rarely cause cardiac tamponade.
Painful recurrences of pericarditis often respond to NSAIDs but may require corticosteroids. Increasing evidence suggests that colchicine (0.5 mg orally twice a day for 6-12 months with a gradual taper) should be used with NSAIDs before attempting steroid therapy. In the Colchicine for Recurrent Pericarditis (CORE) trial, adjunctive colchicine significantly decreased symptom persistence at 72 hours from 31.0% to 9.5% and the recurrence rate from 50.6% to 24.0% at 18 months.12 A number of ongoing multicenter, double-blind randomized trials should clarify the role of colchicine in the treatment and prevention of pericarditis.9 Once steroids are administered, dependency and the development of steroid-induced abnormalities are potential sequelae. Prednisone is begun at a high dose (60-80 mg/d) for at least 4 weeks and tapered slowly over the next 3 months. When necessary, the risks of long-term steroids should be minimized by using the lowest possible dose, alternate-day therapy, combinations with nonsteroidal drugs, or colchicine. In the most difficult cases, relapse occurs every time the dose of prednisone is reduced below 5 to 20 mg/d. When this occurs, the patient should be maintained for several weeks on the lowest suppressive dose before the next taper commences. In addition to its use as an adjunct to corticosteroid therapy, colchicine may be used as monotherapy for the prevention of recurrent pericarditis. Intrapericardial administration of triamcinolone (300 mg/m2) has been shown to relieve symptoms in patients with recurrent autoreactive myopericarditis,10 and azathioprine (50-100 mg/d) has also been used to prevent recurrent episodes. Although encouraging results have been reported in a series of patients who underwent pericardiectomy for recurrent pericarditis, pericardiectomy may simply abbreviate rather than terminate the painful recurrences.1 Thus, pericardiectomy should be considered only when repeated attempts at medical treatment have clearly failed.
Pericardial Effusion
Accumulation of transudate, exudate, or blood in the pericardial sac, a common complication of pericardial disease, should be sought in all patients with acute pericarditis. Pericardial effusions are reported to be associated with heart failure, valvular disease, and MI in 14%, 21%, and 15% of cases, respectively. Hydropericardium results from elevated right atrial pressure, and limited venous and lymphatic drainage from the pericardium are the usual explanations for effusions associated with heart failure and LV hypertrophy, respectively.
Pericardial effusions are common after cardiac surgery. In 122 consecutive patients studied before and serially after cardiac surgery, effusions were present in 103 patients; the majority appeared by postoperative day 2, reached their maximum size by postoperative day 10, and usually resolved without sequelae within the first postoperative month. However, large effusions or effusions causing pericardial tamponade are uncommon following cardiothoracic surgery. In one retrospective survey of >4500 postoperative patients, only 48 were found to have moderate or large effusions by echocardiography; of those, 36 met diagnostic criteria for tamponade.13 Use of preoperative anticoagulants, valve surgery, and female sex were all associated with a higher incidence of tamponade. Symptoms and physical findings of significant postoperative pericardial effusions are frequently nonspecific, and echocardiographic detection and echo-guided pericardiocentesis, when necessary, are safe and effective; prolonged catheter drainage reduces the recurrence rate.14 Pericardial effusions in cardiac transplantation patients are associated with an increased incidence of acute rejection.
Chronic effusive pericarditis is an entity of unknown etiology that may be associated with large, asymptomatic effusions. Many conditions that cause pericarditis (eg, uremia, tuberculosis, neoplasia, connective tissue disease) produce chronic pericardial effusions.
Characteristics of the pericardial fluid other than culture and cytology are usually too nonspecific to be of diagnostic value. In a recent retrospective study of 173 patients who had undergone pericardiocentesis, no biochemical or cell count parameter accurately differentiated the various causes of effusion (eg, idiopathic, neoplastic, or acute pericarditis).15 However, in another retrospective series, one-fifth of the patients had a specific etiologic diagnosis that had implications for management and prognosis. Moreover, in certain situations, it is mandatory to determine the nature of the pericardial fluid. For example, in patients with neoplastic disease, it is important to determine whether pericardial effusion indicates invasion of the pericardium or a complication of radiation therapy. Cytologic examination of the fluid is also important in cases in which the primary tumor has not been identified clearly. In cases of bacterial or other nonviral infections, it becomes necessary to discover whether the pericardial effusion is exudative and to culture pericardial fluid; this is particularly important when tuberculous or fungal pericarditis is suspected. Transudative effusions (hydropericardium) occur in heart failure and other states associated with chronic salt and water retention (including pregnancy), and exudative effusions occur in a large number of the infectious and inflammatory causes of pericarditis. Although frank hemorrhagic effusions suggest recent intrapericardial bleeding, sanguineous and serosanguineous effusions occur in many infectious and inflammatory disorders. In certain disorders, the nature of the pericardial fluid has greater diagnostic value. For example, chylous pericarditis implies injury or obstruction to the thoracic duct, and cholesterol pericarditis is either idiopathic or associated with hypothyroidism, rheumatoid arthritis, or tuberculosis.
The etiology of a pericardial effusion may be difficult to determine on historical or clinical grounds. In one series of 322 patients admitted to a tertiary care hospital with at least moderate effusions, the cause of the effusion was attributed to a preexisting medical condition in 192 patients. In the remaining patients, those with inflammatory signs were more likely to have acute idiopathic pericarditis; those without inflammatory signs or tamponade were more likely to have chronic, idiopathic effusions; and those presenting with tamponade but without inflammatory signs were more likely to have a malignant effusion.16
Although specific diagnoses are possible using visual, cytologic, and immunologic analysis of the pericardial effusion and pericardioscopic-guided biopsy, there are clinical situations in which it is unnecessary to obtain pericardial fluid for analysis. For example, when pericardial effusion is found in a patient with typical viral or idiopathic pericarditis, pericardiocentesis should not be considered unless the effusion fails to respond to anti-inflammatory treatment or cardiac tamponade develops. Similarly, when a patient undergoing chronic hemodialysis develops pericardial effusion, examination of pericardial fluid is needed only when the clinical course suggests a different etiology or when hemodynamic compromise is suspected.
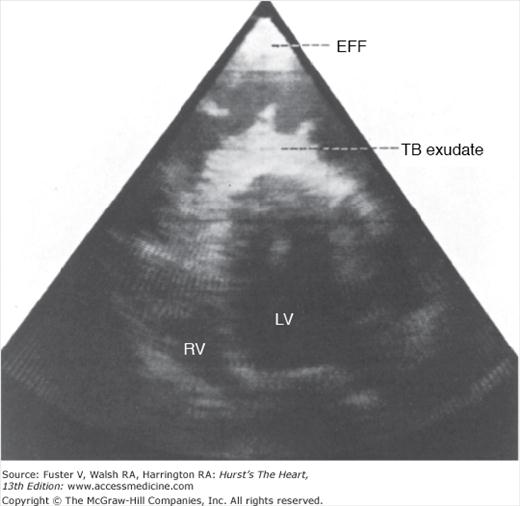
Echocardiography is the procedure of choice for the diagnosis of pericardial effusion. Although flask-shaped enlargement of the cardiac silhouette on chest radiography occurs with a moderate or large pericardial effusion (see Fig. 85–5), differentiation of large effusions from cardiac dilatation often is difficult or impossible. In contrast, the relative contributions of cardiac enlargement and pericardial effusion to overall cardiac enlargement and the relative roles of tamponade and myocardial dysfunction to altered hemodynamics can be evaluated with echocardiography. Attention to technical detail results in excellent sensitivity and specificity. The diagnostic feature on M-mode echocardiography is the persistence of an echo-free space between parietal and visceral pericardium throughout the cardiac cycle (see Fig. 85–6). Separations that are observed only in systole represent clinically insignificant accumulations. Two-dimensional echocardiography (Fig. 85–7) has superior spatial orientation and allows delineation of the size and distribution of pericardial effusion, as well as detection of loculated fluid. As the amount of pericardial fluid increases, fluid distributes from the posterobasilar LV apically and anteriorly, and then laterally and posteriorly to the left atrium. Pericardial effusions are described as small, moderate, or large based on the size of the echo-free space seen between the parietal and visceral pleurae on two-dimensional echocardiography. Fluid adjacent to the right atrium is an early sign of pericardial effusion. Frond-like, band-like, or shaggy intrapericardial echoes should alert one to the possibility of a difficult and potentially less therapeutic pericardiocentesis (Fig. 85–8) but have little value in identifying the cause of the effusion. Epicardial fat tissue is more prominent anteriorly but may appear circumferentially, thus mimicking effusion (lipid envelope). Fat is slightly echogenic and tends to move in concert with the heart, two characteristics that help distinguish it from an effusion, which is generally echolucent and motionless; however, these characteristics may be difficult to discern. In addition to its mimicry, pericardial fat accumulation is a source of bioactive molecules, is significantly associated with obesity-related insulin resistance, and is a coronary risk factor.17 Pericardial fat correlates with LV mass, impaired diastolic function, and atrial enlargement, but the associations are attenuated when visceral abdominal fat are taken into account.18
Pericardial effusions are easily detected by computed tomography (CT) (Fig. 85–9). The size, geometry, and distribution of pericardial effusions can be obtained with this technique, and the attenuation coefficients for blood, exudate, chyle, and serous fluid are generally sufficiently characteristic to identify the nature of the effusion. CT may be useful in estimating the hematocrit of the pericardial effusion,19 identifying loculated pericardial effusions, and guiding pericardiocentesis. Loculated and recurrent pericardial effusions can be treated safely and effectively with video-assisted thoracoscopic pericardial fenestration.
Magnetic resonance imaging (MRI) detects pericardial effusion with high sensitivity and provides an estimate of pericardial fluid volume; in addition, it effectively detects loculated pericardial effusion and pericardial thickening. Inflamed pericardium and adhesions have high signal intensity relative to pericardial fluid and myocardium, providing a potential means of identifying the nature of the effusion.
Drainage of a pericardial effusion is usually unnecessary unless purulent pericarditis is suspected or cardiac tamponade supervenes, although pericardiocentesis is sometimes needed to establish the etiology of a hemodynamically insignificant pericardial effusion. Persistent (>3 months) large or progressive effusion, particularly when the cause is uncertain, also warrants pericardiocentesis.20 However, routine drainage of a large pericardial effusion without tamponade or suspected purulent pericarditis has a low diagnostic yield (7%) and no clear therapeutic benefit. Anticoagulants should be discontinued temporarily, if possible, to reduce the risk of cardiac tamponade. In patients on chronic oral anticoagulation, heparin should be used because its effect can be reversed rapidly. The management of anticoagulation in patients with acute pericarditis and effusion requires careful analysis of the competing risks and benefits because little objective data are available for decision making. Acute pericarditis generally responds to NSAIDs within a few days, during which time anticoagulants may be withheld in low-risk patients; patients at higher thromboembolic risk can be switched to heparin while awaiting a clinical response. Unexplained effusions should be thoroughly evaluated to exclude hemorrhage. Large effusions may respond to NSAIDs, corticosteroids, or colchicine. Specific treatment for pericardial effusion is considered later in this chapter (see Specific Forms of Pericardial Heart Disease).
Cardiac Tamponade
Cardiac tamponade is a hemodynamic condition characterized by equal elevation of atrial and pericardial pressures, an exaggerated inspiratory decrease in arterial systolic pressure (pulsus paradoxus), and arterial hypotension. Arterial hypotension is generally a late sign in chronic effusions, and occasionally, a heightened sympathoadrenal state produces systemic hypertension. As intrapericardial pressure rises, venous pressures increase to maintain cardiac filling and prevent collapse of the cardiac chambers. Although the absolute intracardiac pressures are elevated, the transmural pressures—that is, cavitary diastolic pressure minus pericardial pressure—are practically zero or even negative. The greatly reduced preload is responsible for the decrease in cardiac output and, when compensatory mechanisms are exhausted, arterial pressure decreases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree