Previous terms and eponyms
Replaced by newer terms
Superficial femoral vein
Femoral vein
Greater or long saphenous vein
Great saphenous vein (GSV)
Lesser or short saphenous vein
Small saphenous vein (SSV)
Saphenofemoral junction
Confluence of the superficial inguinal veins
Giacomini vein
Intersaphenous vein
Posterior arch vein or Leonardo’s vein
Posterior accessory great saphenous vein of the leg
Cockett perforators (I, II, III)
Posterior tibial perforators (lower, middle, upper)
Boyd’s perforator
Paratibial perforator (proximal)
Sherman’s perforators
Paratibial perforators
“24 cm” perforators
Paratibial perforators
Hunter’s and Dodd’s perforators
Perforators of the femoral canal
May’s or Kuster’s perforators
Ankle perforators
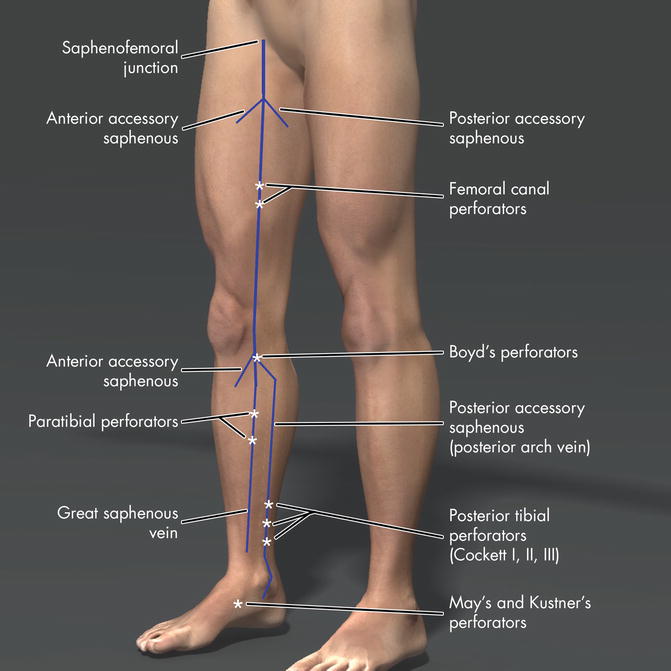
Fig. 14.1
Anatomy of the major perforator veins in the lower limb
The paratibial perforators connect the great saphenous vein to the posterior tibial veins. Multiple paratibial perforators are found 2–4 cm posterior to the medial edge of the tibia or “Linton’s Lane” and are particularly important for conducting a proper SEPS procedure. The perforators of the femoral canal (previously referred to as Dodd and Hunterian perforators) connect the great saphenous and femoral veins.
Ankle perforators include the former May’s or Kuster’s perforators. In the foot, there are dorsal plantar, medial, and lateral foot perforators where the normal direction of flow is outward, distinctly opposite from PVs in the calf. The large perforator in the foot arises between the first and second metatarsal bones and connects the pedal vein to the superficial dorsal venous arch.
14.4 Pathophysiology
PVs alone do not appear to be the primary cause of venous ulcers. Instead, they are almost always accompanied by local or axial superficial and/ or deep venous reflux or obstructive disease. Although PVs are frequently found in areas of intense inflammation, pre-ulcerative skin changes or in the vicinity of ulcers, they are not found as isolated abnormalities in venous ulcers [15]. Frequently, the most recalcitrant ulcers are associated with reflux in all three systems (deep, superficial and PVs). Neither isolated perforator nor isolated deep venous reflux is commonly found associated with severe CVD [16].
Usually two or more venous systems are abnormal in advanced CVD. PVs appear to act as reentry points between two axial systems allowing blood to flow from incompetent superficial to deep or vise versa [17]. If the primary problem is deep venous obstruction or reflux, the elevated venous pressure produced by deep venous obstruction or reflux during calf muscle contraction is transmitted to the connecting perforators and into the superficial veins. The blood under the calf muscle pump is forced to escape via the PVs and “yo-yos” up and down the deep system [17]. This may result in enlargement of the dermal capillary bed and release of proteins into the interstitial space including fibrinogen, which may eventually result in ulceration [18, 19].
In primary venous insufficiency with no prior DVT, the pathology is likely a refluxing saphenous system causing dilatation of the PVs, rendering the valves incompetent and often referred to as a “reentry perforator”. This is supported by the findings of Stuart and Campbell who found that in cases of combined PVs and saphenous reflux, by abolishing the superficial saphenous vein alone PVs were no longer detectable or became competent [20, 21]. In a prospective study by Labropoulos and colleagues, new perforator incompetence always occurred with reflux in the superficial veins [22]. If the clinical state worsened, outcomes could not be attributed to development of PVS alone because of the inevitable presence of superficial disease [22].
Increasing size and numbers of PVs are associated with increasing severity of CVD [23, 24]. Size of PVs play an important role since larger diameters of PVs are more likely to be incompetent [25]. Diameters of >3.5mm are associated with reflux in 90% of cases [26]. PVs with diameters >3.9mm possess a high specificity of 96%, but lower sensitivity of 73% for incompetence with the lower sensitivity attributable to one third of incompetent PVs possessing diameters of <3.9mm [22]. The observation that increasing numbers of PVs lead to increasing severity of CVD is supported by the fact that higher numbers of PVs produce higher venous filling indices [23, 24].
14.5 Evidence in Favor of Importance of PVs
The clinical importance of PVs is supported by the fact that ulceration and skin stasis changes inevitably occur in the “gaiter area” between the distal point of the soleus muscle to the ankle where most large incompetent PVs are found. In ulcer disease, 50–60% of patients have incompetent perforators, and as the limb becomes more severely symptomatic, the association of PVs with either superficial or deep vein reflux or obstruction increases [24, 28]. The prevalence of PVs increases with clinical severity stratified by the CEAP classification, and they increase with the prevalence of deep vein reflux [16, 29].
Clinical evidence supporting the importance of PVs are found in studies treating the more severely symptomatic groups of C4–C6. Although there are no RCT’s proving its importance, the best data at the time of this publication consists of one large multicenter registry and several observational studies.
The North American Subfascial Endoscopic Perforator Surgery registry (NASEPS) consisted of 155 limbs, collected from 17 US centers, in which 85% were C5–6 [30]. When treated with SEPS, median time to ulcer healing was 54 days; 88% healed at 1 year and 72% remained healed by 2 years. However 71% had concomitant saphenous stripping with SEPS and benefit of SEPS could not be attributed to treating perforators alone. SEPS was appealing since it was associated with low wound complication rate of 6%, much improved over the more invasive Linton procedure. Since most interventions including treatment of superficial reflux, the direct impact of treating PVs alone could not be clearly distinguished from the important effect of treating the superficial axial system.
Several observational studies suggest long term benefit of PV treatment for venous ulceration. Iafrati reported the long term outcome of C5–C6 disease in 35 cases of saphenous or variceal surgery plus SEPS, and 16 cases of SEPS alone in which early ulcer healing rate of 74% at 6 months [31]. Ulcer recurrence was only 13% at 5 years, and best results were associated with GSV stripping, primary venous insufficiency and ulcer <2 cm.
In another long-term follow up study of 9 years, Tawes reported on their retrospective multicenter experience of 832 patients with C4–6 disease undergoing SEPS [32]. Although 55% had stripping plus SEPS, 92% healed their ulcer with a recurrence rate of 4%. Finally, in a study of SEPS and saphenous stripping, healing of C6 cases occurred in 91% by mean of 2.9 months, with an ulcer recurrence of 6% at 30 months [33].
A meta-analysis of SEPS by Luebke found that for severe CVD, SEPs showed early benefit with rapid ulcer healing and decreased ulcer recurrence [34]. They concluded that SEPS in contrast to the Linton procedure was safer, with fewer complications. In another systematic review of 20 studies (one RCT comparing endoscopic to open perforator interruption and 19 case series), Tenbrook and colleagues report early ulcer healing in 88% and recurrence in 13% at 21 months. [35]. But again, this report included studies with both saphenous intervention and SEPS.
In an attempt to isolate the effect of sclerotherapy on perforators alone from treatment of superficial disease, the study from Straub Clinic & Hospital excluded those who had received treatment of the superficial system up to 2 years prior to ultrasound-guided sclerotherapy (UGS) of perforators [36]. The intent was to remove the concomitant confounding effects of treating the GSV and superficial veins. In all 80 limbs in which only the perforators were treated, successful ablation was achieved in 75% at 20.1 month follow-up. Eighteen percent had preexisting deep or superficial axial reflux. In C4–C6 patients, Venous Clincal Severity Score (VCSS) and Venous Disability Score (VDS) significantly improved. Of 37 limbs with ulcers, 86.5% showed rapid healing of ulcers by mean of 35.6 days, Ulcer recurrence was noted in 32.4% after single treatment, which was reduced to 13.5% after a second treatment despite low compliance stocking use of 15%. Recurrence appeared to be related to new or recurrent perforators and post-thrombotic disease [36].
Proof of importance of PV is supported by hemodynamic abnormality in the pathologic state. Leg perforators are associated with abnormal ambulatory venous pressures well above 100 mm Hg during calf muscle contractions. The pressure is released through the PVs from deep to superficial veins with calf contraction analogous to the “broken bellows” described by Negus and Friedgood [37]. Zukowski and Nicolaides showed that 70% of those with ulcerations have moderated to severe hemodynamically significant perforators by ambulatory venous pressure testing [38].
Correction of hemodynamic abnormality has been observed with correction of PVs and is supported by several small studies. Padberg showed ablation of superficial and PVs in 11 cases resulted in improved expulsion fraction and half refill times with no ulcer recurrence when examined by air plethysmograph, foot volumetry and duplex scanning at a mean of 66 months [39]. Rhodes et al. reported significant improvement in calf muscle pump function and vein competence assessed by strain gauge plethysmograhy in 31 limbs following SEPS. Seven underwent SEPS alone and the remaining underwent SEPS plus stripping [40].
14.6 Evidence Against the Importance of IPVs
Isolated incompetent PVs are rare (reported in 3–8 % of CVI patients) [41, 42]. Therefore, separating the effects of isolated IPVs from the effects of superficial or deep venous pathology with respect to pathophysiology and response to treatment has been challenging [43]. To address this important issue, randomized controlled trials (RCTs) have been conducted to measure the effect of IPV treatment on superficial venous treatment by randomizing the groups with or without SEPS.
In mild CVD, abnormalities of the superficial venous system appear to be of greater clinical significance than perforator disease. Two RCTs have shown that with non-ulcer patients, the addition of surgical treatment of IPVs did not impact the clinical results of treating the superficial system alone [44, 45]. Kianiford and colleagues compared stripping of the GSV with or without SEPS and showed no benefit to adding perforator surgery to the GSV treatment [44]. These results were supported by the findings of Fitridge et al. who randomized stripping of the GSV with or without open interruption of previously marked IPVs and found no physiologic benefit (as assessed by air plethysmography) of adding IPV treatment [45]. Superficial axial reflux appeared to show a greater independent contribution toward venous symptoms in uncomplicated disease than IPVs. This is also supported by findings that in cases of both superficial and perforator disease, stripping of the saphenous system from the groin to the knee led to either reversal incompetence in PVs or complete “elimination” of the PVs in 50–80% probably by removing the venous outflow tract. Not only did number of PVs diminish but size of PVs was also reduced [20, 21, 46, 47].
In contrast to mild CVD, evidence for IPV surgery is less clear with clinical, etiologic, anatomic, pathophysiological (CEAP) classes C4–6. With regards to ulceration, a RCT published by the Swedish SEPS group summarized by Nelzen et al., the early results of their trial comparing saphenous surgery with or without SEPS and demonstrating that at 1-year follow-up adding SEPS did not make a difference in mean time to ulcer healing or recurrence [48]. However, the study was limited by the investigators’ inability to accrue the targeted number of patients and was therefore underpowered. It was further limited by the short duration of follow-up. Longer follow-up is needed to establish the effect, if any, that SEPS may have had on healing and ulcer recurrence.
There are two RCTs that did not control for the presence of concomitant GSV surgery and suggested perforator vein surgery had no advantage over compression therapy for ulcers [49, 50]. Stacey et al. examined the effect of IPV ligation on ulcer recurrence in CEAP class C5 patients [49]. They compared IPV ligation combined with saphenous vein surgery with external compression alone and found no hemodynamic advantage in either group, except that those with primary valvular insufficiency (not postthrombotic) had better improvement in calf muscle pump function. The second RCT, by van Gent et al., also suggested no benefit from IPV surgery over compression, although 54 % had concomitant GSV surgery [50]. Despite the limitation that both studies included concomitant GSV surgery, one would have anticipated that adding GSV surgery should have benefitted the IPV surgical group since we know that superficial surgery is superior to compression alone with respect at least in regard to reducing ulcer recurrence [51, 52, 53].
Lastly, hemodynamic studies cannot differentiate the contribution of isolated PVs from those with associated deep or superficial axial reflux which is further confounded by the fact that isolated PVs are rare [22]. Another point to be made against the importance of IPVs is that normal limbs have outward flow in the perforator veins up to 21 % and not all ulcers are associated with incompetent perforator veins [13]. Up to 40 % of venous ulcers have no perforator involvement at all. When IPV is present it is almost always associated with incompetent superficial and/or deep veins [41]. Published evidence that hemodynamic parameters do not improve after IPV ligation have supported the lack of importance of IPVs [49, 54].
14.7 Fate of IPV’s After Surgery
PVs will regress afer surgery but increase again with time, thought to be the result of redistribution of venous flow [44]. In a report by van Rij, the majority (76%) of patients developed a new or recurrent PVs after GSV stripping to the knee and direct perforator ligation at 3 years, in stark contrast to the 21% reported after SEPS [55, 56]. The small Dutch group led by Sybrandy reported that after open Linton procedure or SEPS, perforator recurrence rate was 40% at 48 months [57]. Although PV’s are associated with recurrence, what remains unclear is whether they are the cause of recurrence. The REVAS group (recurrent varices after surgery) published the experience of eight countries with superficial reflux and previous superficial surgery, and although 55% were associated with incompetent perforators, cause of recurrent symptoms could not be clearly attributed to the perforators [58].
14.8 Diagnosis
Duplex scanning of PVs is best accomplished with the patient in either the reverse Trendelenburg position or standing with the weight placed on the opposite limb. Perforator vein incompetence is defined as the presence of outward or bidirectional flow which can be elicited by manual proximal and distal compression with rapid release, with active dorsiflexion and/or standard rapid cuff release in the standing position with the weight on the opposite limb [59]. Flow lasting greater than 0.5 s in either outward or bidirectional flow is considered abnormal. Pathologic perforator veins must be 3.5 mm or more in diameter based on correlation with clinical severity in the previously mentioned trials [25, 23]. Diameter of the perforator vein is best measured at the level of the fascia. In the case of dividing perforator veins, the measurement is taken away from the division above the fascia to avoid overestimation of the width of the vein.
The optimal method to identify PVs is to scan the GSV first, followed by the posterior accessory GSV of the calf, and then any major tributaries in the calf. Attention should be paid to the presence of skin changes: large tributaries may be clustered in the area that could represent a termination point into the perforator vein. The presence of an ulcer or dressing should not be a deterrent to scanning, as this may be the site of a clinically important perforator. If reflux is detected in the deep vein or superficial vein below a competent valve, it is important to localize the perforator of the femoral canal, which usually connects with a distal incompetent GSV. If reflux is seen in the popliteal vein only, the usual source and point of retrograde outflow is the SSV. The most common IPVs are the posterior tibial perforators middle and upper, which communicate with the posterior accessory GSV of the calf, and the paratibial perforators in the proximal calf, which communicate with the GSV.
Venography is an uncommon method of interrogating perforator veins and has largely been replaced by duplex scanning. Historically, venography was the only method of examining perforators during a time when perforators were being associated with ulcers and treatment by open surgical elimination was widely practiced. The details are well described by Kamida et al. [60]. In brief, to examine perforator veins venographically, a small 22 gauge butterfly needle is inserted into a dorsal foot superficial vein. The exam is best performed in the upright, non-weight-bearing position by having the patient stand with the contralateral leg on a box. Ankle tourniquets are essential to drive the contrast into the deep system and evaluate for perforating veins. The tourniquets are placed at various levels in the leg to identify points of communication between the deep and superficial veins. Fluoroscopic examination of the pattern of venous filling is essential part of identifying the presumably pathologic perforators.
14.9 Treatment Options and Techniques
Current options for treatment are SEPS, direct open surgical division of individual perforators, thermal ablation with either radiofrequency ablation (RFA) or endovenous laser ablation (EVLA), or ultrasound-guided sclerotherapy (UGS).
14.9.1 SEPS
After Hauer described the endoscopic procedure for IPV, O’Donnell introduced the application of the laparoscope to facilitate its technical needs [61]. Gloviczki and colleagues and Conrad are to be credited for introducing the CO2 inflation method of creating the dissecting space [62, 63]. Standard laparoscopic equipment is required and either the single or double port technique could be used. If the double port method is selected, the 5 mm distal port to pass the 5 mm harmonic scalpel, scissors, or dissecting instruments and a 10 mm proximal port with 10 mm camera are set up. The leg is exsanguinated with an Esmarch bandage and proximal thigh tourniquet inflated to 300 mmHg. Balloon dissection is performed with pressures of 30 mmHg. The proximal port is placed 10 cm distal to tibial tuberosity; distal port is placed 10–12 cm further down but above the medial ankle or diseased gaiter area.
For best results, Rhodes and colleagues recommend paratibial fasciotomy to ligate the middle and upper posterior tibial perforators in the intermuscular septum [64]. Care is taken to place the fasciotomy close to the tibia to avoid injury to the posterior tibial vessels and nerve. The retromalleolar lower posterior tibial perforator is best treated by small incision directly over it or ultrasound-guided foam or liquid sclerotherapy. If treatment of the superficial axial system is required, the ablation or stripping and phlebectomy are performed following the SEPS procedure.
14.9.2 Percutaneous Ablation
Percutaneous ablation techniques include radiofrequency ablation (RFA), endovenous laser ablation (EVLA), and ultrasound-guided sclerotherapy (UGS). Percutaneous ablation allows precise identification and localization of each perforator vein that can provide treatment without disruptive incisions or tissue dissections. It can be done in the outpatient setting; local (RFA, EVLA) or no (UGS) anesthesia is necessary, and it can be used as an adjunct procedure during surgery for CVD. It is beneficial in cases where the overlying skin is severely sclerotic or with the presence of an active ulcer. Percutaneous ablation is also helpful in patients who are obese or poor candidates for SEPS due to anesthesia risks. These procedures can be repeated without sequelae.
With percutaneous ablation, it is imperative to identify the perforator artery (Figs. 14.2, 14.3, 14.4, 14.5, and 14.6). “Blind sticks” are discouraged due to the significant risk of inadvertently ablating the perforator artery, which could lead to skin necrosis. While injecting the vein under duplex guidance, occasionally, resistance is encountered which could indicate the needle is now outside the vein or the vein is maximally filled, at which time the flow can appear stagnant during the injection. At that point, ablation must be stopped and the duplex used to check access for PV patency and color flow. Alternatively, some advocate injecting or ablating the superficial vein into which the incompetent perforator vein drains. Finally, good results have been obtained with UGS by injecting the microvasculature associated with IPV skin changes.
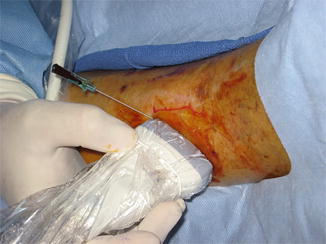
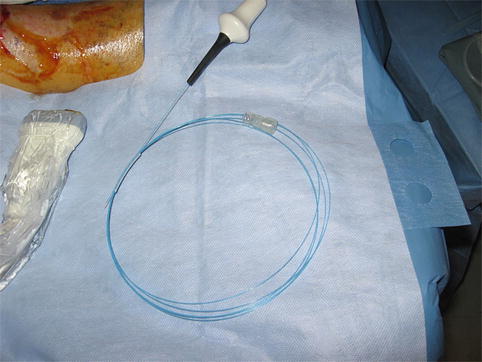
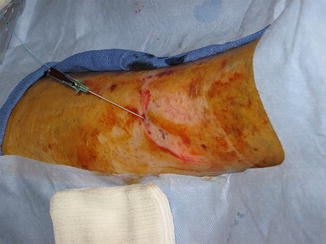

Fig. 14.2
Importance of identifying the perforator artery begins with confirming Doppler data with image. Initially perforator is identified with typical to and fro flow

Fig. 14.3
Perforator artery adjacent to vein is clearly identified by arterial signal

Fig. 14.4
Perforator artery is avoided and not in the path of the needle while access of vein is achieved

Fig. 14.5
Successful ablation of perforator vein
Percutaneous ablation is generally confirmed when there is no spontaneous flow and no flow with proximal and distal compression and release. If there is persistent flow through the PV, reinjection with the same technique can be done either at the same site or through a superficial vein communicating with the PV since often times reaccessing a previously treated PV can be difficult. Inadvertent infiltration of the perivascular tissue during UGS at standard volumes usually results in no major consequences unless the perforator artery is injected.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


