Fig. 11.1
Relative position of patient and sclerotherapist when done efficiently
Fig. 11.2
Example of a poor ergonomic position
In some practices, the skin of the entire leg is prepped with alcohol prior to the sclerotherapy session; in others, an alcohol prep is used locally before each injection; and in still others, no prep is used at all. Infection is extremely uncommon following sclerotherapy, so practitioners should decide with which method they are most comfortable. According to infectious disease specialists, patients with foreign body implants, or those with underlying medical conditions that require antibiotic prophylaxis prior to invasive surgery or dental work, do not need such treatment prior to sclerotherapy.
To facilitate injections, along with stretching of the skin, a small gauge needle, such as 30–33 gauge, may be angled to augment access that is near parallel rather than perpendicular (Fig. 11.3). In order to limit side effects of telangiectatic matting and hyperpigmentation, it is generally considered better to make numerous injections using small volumes of sclerosant in larger syringes so the pressure generated is low (e.g., it is possible to generate much greater pressure with a TB syringe than a 3 or 5 mL syringe). Limiting each injection treatment area to 2–4 cm2 may also reduce matting and hyperpigmentation.
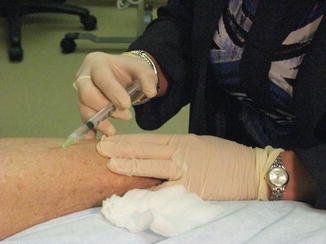

Fig. 11.3
To facilitate injections, along with stretching of the skin, a small gauge needle, such as 30–33 gauge, may be angled to augment access that is near parallel rather than perpendicular
11.4 From Liquid to Foam Sclerotherapy
Ultrasound-guided liquid sclerotherapy is considered by many to be ineffective in causing permanent vein sclerosis in large veins [7]. However, utilization of foam for large truncal veins has fundamentally changed conventional wisdom regarding successful chemical ablation of saphenous and large tributary veins. Good midterm results have been reported following duplex-guided chemical ablation using foamed detergent sclerosants, compared to results from thermal and surgical ablation [8–10].
Foaming a detergent sclerosing agent changes the biological activity of the drug. While most would consider foaming a sclerosant to be an “off-label” use of an approved sclerosant, the drug in its foam form could be considered by regulatory agencies as unapproved. Of more practical importance is that a foamed sclerosant is much more potent than in liquid form, requiring less volume and concentration than in liquid form to successfully achieve vein sclerosis [11–13].
Side effects and complications of foam injection include sequestered coagulum, hyperpigmentation, telangiectatic matting, headache, visual disturbances, chest discomfort, and changes in neurologic or mental status [14].
11.4.1 Methods of Foam Production
Foam is produced by agitating a gas with a liquid detergent sclerosing agent, most commonly via a straight connector (Fig. 11.4) or three-way tap (Fig. 11.5), between two syringes containing the ratio of four parts gas to one part liquid, described by Tessari in 2000 [15]. Twenty to forty agitation cycles will produce durable microbubble foam.



Fig. 11.4
Example of a straight connector

Fig. 11.5
Example of a three-way tap
Foam produced for injection should be durable enough to allow adequate time for injection of the microbubbles but should also break down quickly once injected to reduce the effects of gas embolization. Sterile or room air has been most commonly employed, but a more biocompatible gas such as carbon dioxide (CO2) or a gas combining carbon dioxide and oxygen (O2) may lead to fewer side effects due to greater solubility and less in vivo stability than air (limited gas embolization) [16–18].
The detergents used to create foam for sclerosis are sodium morrhuate, ethanolamine oleate, sodium tetradecyl sulfate (STS), and polidocanol, though most phlebologists utilize either polidocanol or STS. Foam can be prepared in various concentrations to treat small or large veins and can be instilled via indwelling catheter or by direct needle puncture [18, 19].
Ultrasound-guided foam sclerotherapy is usually performed by the practitioner alone, but the aid of an assistant to generate foam or a sonographer to image the target vein may make the entire procedure more efficient and time-effective. For the indwelling catheter method, the sclerotherapist can easily produce high-quality (small bubble size) foam and inject it into the target vein quickly before foam degradation occurs (large bubbles, lower efficacy, greater risk of adverse events). For the direct needle injection method, however, enlisting the help of an assistant or sonographer is useful (Fig. 11.6).
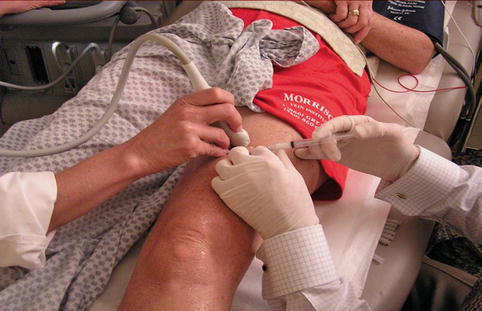

Fig. 11.6
For the direct needle injection method, however, enlisting the help of an assistant or sonographer is useful
Imaging of the target vein may be in the longitudinal view (Fig. 11.7) or transverse view (Fig. 11.8). With the direct needle injection method, a transversely imaged target vein will make access easier. But for the catheter method, the longitudinal view may be more advantageous for cannulation. In Fig. 11.9 we see the technique of advancing the needle or catheter along the long axis of the ultrasound probe so that the needle is visualized all the way from the skin insertion site to the target vein. This is different than the “triangulation” technique typically favored by radiologists, wherein the needle is inserted along the short axis of the probe allowing visualization of the tip only when it arrives at the target vein (Fig. 11.10).
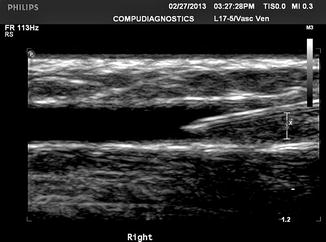
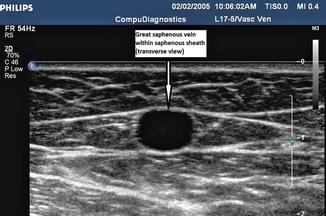
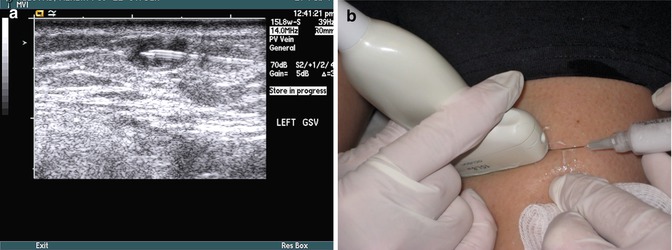

Fig. 11.7
Longitudinal view of target vein

Fig. 11.8
Transverse view of target vein

Fig. 11.9
Advancing the needle along the long axis of the ultrasound probe. (a) ultrasound image of needle course into target vein and (b) visual image of injection technique

Fig. 11.10
Inserting the needle along the short axis of the ultrasound probe
The volume of foam injected at each site is determined by the size of the treatment vein and the volume required to replace blood in the vein with foam but is usually limited to 0.5–3 mL. The total volume of foam used per treatment session is the subject of debate, as little data has been published regarding the volume of foam necessary to achieve successful vein sclerosis while limiting side effects. The upper limit of foam is generally considered to be 10 mL when utilizing air-based foam, while there is evidence that the use of biocompatible gas to produce foam allows for higher volumes to be injected safely [16, 20].
11.4.2 Efficacy of Foam Sclerotherapy
Efficacy trials have been conducted internationally [8, 21–26]. Successful ablation has been reported to range from 68 to 100 %, with follow-up from 1 month to 10 years, though interpretation of these results is difficult because of the differences in definitions of success, the use of surrogate markers (occlusion or narrowing of the treated vein, resolution of reflux), differing primary outcome markers (resolution of symptoms, improved quality of life scores, recurrent varices, ulcer healing), and the number of ultrasound-guided foam sclerotherapy sessions needed to achieve success, among others. Recently a consensus document has been published under the auspices of the Union Internationale de Phlebologie (UIP) in an attempt to standardize duplex reporting following treatment of lower extremity venous disorders [27].
It is also important to state that simply creating thrombosis of a target vein will likely not result in permanent occlusion of the vein. Damage to or destruction of the vein wall is necessary to ensure sclerosis [28]. It may be necessary to produce injury through the intimal layer into the media in order to achieve the desired destruction [29].
11.4.3 Methods to Improve Foam Sclerotherapy Efficacy
Various methods have been proposed to improve efficacy: agitation methods which enhance the durability and uniformity of foam [18, 30, 31], increased sclerosant concentration [22] or volume used [22, 32], indwelling catheter method [18, 33], foam production methods resulting in smallest bubble size possible [18], and leg elevation (for “empty vein”) [34]. In spite of a number of well-conducted studies [22, 25, 35, 36] in a review of the published and unpublished data available in the world literature, Jia et al. [14] concluded that there exists insufficient data to determine the optimal volume of foam, optimal concentration, and optimal foam-producing method.
Recently, a method of catheter-directed foam sclerotherapy utilizing ultrasound-guided perivenous tumescent injection has gained interest for reported better efficacy [18, 33].
By increasing the direct contact of the sclerosing agent with the endothelium, foam production methods that create microbubbles of smaller size may add to the efficacy: first, by displacing blood as much as possible from the targeted vein and second, by greatly increasing the total surface area of the smaller bubbles to which the active sclerosant is attached, thereby increasing endothelial contact [23, 37, 38].
For similar reasons, leg elevation prior to the injection will also help clear blood from the vein, thus allowing greater sclerosant contact with the endothelium and less sclerosant mixing with and deactivation by blood.
11.4.4 Safety of Foam Sclerotherapy
Early reports regarding UGFS did not study safety aspects beyond local tissue reactions or venous thrombosis. Primarily because of concern about neurosensory adverse reactions following UGFS [39, 40], more recent reports have looked at such concerns more closely [16, 41–44]. Minor or major complications following foam sclerotherapy have almost uniformly been very limited in incidence and in duration [62]. The practitioner needs to be aware of these risks and their management in order to discuss risk-benefit decisions with patients regarding the use of UGFS.
UGFS may have the potential for pulmonary, visual, and/or cerebral effects, particularly in a patient with a patent foramen ovale, or other right-to-left shunt, which may be more common in patients with varicose veins than in the general population [45] (Table 11.1).
Table 11.1
A list of side effects and adverse events reported to be associated with UGFS
Deep venous thrombosis (DVT) |
Superficial thrombophlebitis (STP) |
Localized perivenous tissue injury |
Paradoxical embolism |
Neurosensory effects |
Respiratory effects |
Deep Venous Thrombosis
Data is lacking on the true incidence of DVT following liquid sclerotherapy. There appears to be a higher incidence of DVT following UGFS than is generally assumed following the use of liquid sclerosants, especially in foam sclerotherapy studies wherein patients are routinely examined for DVT by duplex scanning as opposed to duplex scanning only when warranted by symptoms [9, 22]
However, these thromboses most often involve calf veins that, if followed closely, are most often of limited clinical significance. Symptomatic or femoral-popliteal DVT remain rare, except in the smaller diameter duplicated femoral vein segment [46] or in Myers’ study, veins larger than 5 mm in diameter [47]. There is conflicting in vitro evidence regarding sclerosant foam effect on coagulation [48, 49] and in vivo as well [50]. In the clinical setting, however, Hamel-Desnos has presented evidence that mirrors the author’s experience: foam sclerotherapy can be successfully and safely performed on patients with documented thrombophilia with no increased risk of DVT, given prophylactic anticoagulation [51].
Superficial Thrombophlebitis
Superficial thrombophlebitis probably should be considered a direct consequence of treatment, unless thrombophlebitis extends beyond the region treated or if the inflammation is significantly worse than routinely observed. The incidence varies from less than 1–18 % in the literature probably because of individual interpretation of the clinical findings [52].
Perivenous Tissue Injury
Extravascular tissue injury with the use of UGFS has been reported to be less than 2 % likely because of the benign effects of foam extravasated in the perivenous tissue (significantly lower concentration). This is in contrast to the more damaging effects of some liquid sclerosants (typically three to four times the concentration of foam).
Paradoxical Embolism
A right-to-left shunt, present in 25–30 % of the general population, and perhaps even higher in patients with varicose veins [45], may allow emboli or degradation products released from damaged endothelium to pass to the arterial circulation and affect the microcirculation of any organ. Whether symptoms are related to particulate or bubble emboli or to endothelial destruction products (such as endothelin-1, a potent vasoconstrictor) is currently under investigation. What is known is that UGFS has the potential for nearly always rare and transient pulmonary, cardiac, visual, and/or cerebral effects, particularly in a patient with a patent foramen ovale or other right-to-left shunt.
A rare complication, thromboembolism has also been reported following UGFS when either a thrombus forms in and embolizes from a deep vein or a thrombus extension from a truncal or perforator vein embolizes. In the presence of a right-to-left shunt, the embolus can progress to the arterial circulation with variable sequelae depending on the location of the embolus.
Neurosensory Effects
Reports of significant adverse neurologic events are very few. Forlee et al. reported one case of stroke following varicose vein foam injection sclerotherapy [40]. The patient was subsequently found to have a very large patent foramen ovale. Also reporting neurologic events in patients, Ceulen et al. [39], Bush and colleagues [53], and Ma and Parsi [54] have also reported neurologic events in patients. Because of numerous anecdotal reports of similar events, the true incidence may not be as rare as is reported. The question could be whether all patients should be screened for right-to-left shunt prior to UGFS. The consensus opinion of international experts [55] is that these uncommon neurosensory effects do not justify such pre-sclerotherapy screening, since similar serious incidents have occurred following liquid sclerotherapy, thermal ablation, or surgical stripping [56–58].
It is imperative, however, that the practicing phlebologist has protocols to deal with such serious adverse events should one occur.
Transient visual disturbances, or scotomas, have been mentioned when evaluating adverse events following foam sclerotherapy. Frequencies of occurrence vary from 0 to 6 % following air-based foam injection, with most publications indicating a frequency around 1 % [14, 59]. Other events have been described in the literature. True migraine or ocular migraine is uncommon, but Gillet and colleagues found in a study of 20 patients with visual disturbances following UGFS that clinical features of migraine with aura were present in all patients, suggesting a strong association between visual disturbances following UGFS and migraine [60]. Since it has been established that twice as many patients (50 %) with a history of migraine with aura have right-to-left shunts as is seen in the normal population (25 %) [6, 61], it may be reasonable to expect that patients with a history of migraine with aura have an increased risk of suffering neurosensory events following UGFS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


