Patient Selection
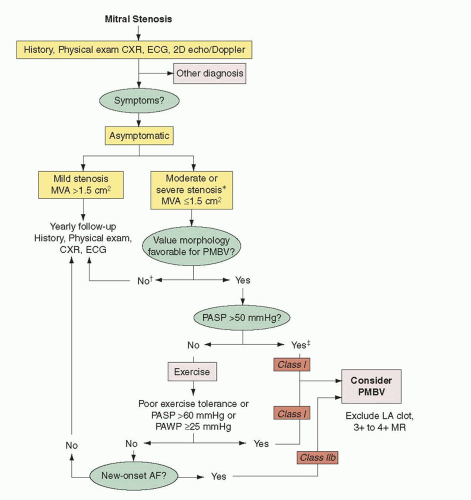
Patients should be selected for percutaneous mitral valvuloplasty based on both clinical and anatomic factors. In general, they should be symptomatic, and mitral valve area as measured by echocardiography and hemodynamics should be <1.5 cm
2. Unlike for valve surgery, the presence of pulmonary hypertension or abnormal left ventricular function is not a contraindication. Patients with anatomically suitable valves who have developed restenosis (commissural refusion) after prior surgical or balloon commissurotomy can also undergo percutaneous mitral valvuloplasty with results almost as good as those for previously untreated patients.
6,
7 Although the procedure can be performed in patients of almost any age, the best clinical results are observed in younger patients, with less predictable long-term results occurring in patients older than 70 years, who are more likely to have deformed and calcified valves. Percutaneous mitral valvuloplasty is a particularly valuable tool in treating the symptomatic pregnant woman with critical mitral stenosis. It can also be a lifesaving emergency procedure in the patient with mitral stenosis and refractory pulmonary edema or cardiogenic shock.
8Asymptomatic patients should be considered for percutaneous mitral commissurotomy when they develop
pulmonary hypertension or new-onset atrial fibrillation.
9 A pulmonary artery peak systolic pressure of >50 mmHg at rest or >60 mmHg with exercise in an otherwise asymptomatic patient represents disease severity that has reached the point where percutaneous commissurotomy should be considered (
Figure 33.1).
9 New atrial fibrillation is less clear an indication but should be considered, especially in patients with mitral valve morphology well suited for percutaneous commissurotomy.
Anatomic Factors in Patient Selection for Balloon Mitral Valvuloplasty
High-quality transthoracic and transesophageal echocardiography (TEE) is an essential part of proper patient selection. TEE prior to the planned valvuloplasty procedure excludes the presence of left atrial thrombus and moderate or severe mitral regurgitation. In addition to ensuring that there are no anatomic contraindications, echocardiography provides valuable information that helps the interventional cardiologist select patients and predict results.
11 The ideal patient has pliable, noncalcified mitral leaflets and mild subvalvular disease. As the degree of subvalvular disease increases, the quality of the result with percutaneous mitral valvuloplasty decreases. Similarly, increasing degrees of calcification of the mitral valve diminish the effectiveness of mitral valve dilatation and increase the complication rate. Dilating mitral valves with commissural calcification may lead to leaflet tearing along noncommissural lines and is associated with a higher incidence of procedure-related mitral regurgitation.
12 Heavy calcification of the valve and/or bicommissural calcification are also associated with poorer acute and long-term outcomes. When commissural fusion is symmetric, even in the presence of calcification, bicommissural splitting is more likely than when commissural fusion is asymmetric.
13,
14Valve deformity increases substantially with age. Older patients who present with mitral stenosis often have valves poorly suited for percutaneous mitral commissurotomy. In such cases, the goals of therapy must be considered individually for patient selection. Patients who are excellent candidates for mitral valve replacement, or those who have associated multivalve or complex coronary disease, may be better served by surgery. The very elderly, or patients with multiple comorbid conditions or prior median sternotomy, may have excellent palliation from percutaneous mitral commissurotomy despite a high degree of valve and subvalvular deformity and calcification. A prototypic example is the octogenarian patient with prior aortic valve replacement and coronary bypass surgery who presents with a heavily calcified mitral valve and severe symptomatic mitral stenosis. The results of percutaneous commissurotomy in these patients are clearly not as good as in younger patients with pliable valves, but the value of palliative therapy is substantial.
Many find the echocardiographic scoring system of Wilkins et al.
15 useful in assessing patients for percutaneous mitral valvuloplasty. This echocardiographic classification system is shown in
Table 33.1. Points are given for leaflet mobility, valve thickening, subvalvular thickening, and valvular calcification. The final score is determined by adding up the points from each category. Higher scores indicate more severe anatomic disease and less likelihood of a successful procedure. The maximum score is 16, and percutaneous mitral commissurotomy results are generally excellent in patients with an echo score of ≤8, indicating favorable anatomy, for example, pliable leaflets, mild or moderate subvalvular disease, and mild or absent valve calcification. A review of over 1,500 patients undergoing balloon mitral valvuloplasty was carried out using a logistic model to improve patient selection.
16 As expected, younger patients with echocardiographic evidence of less severe disease had a better outcome.
Patients with significant valve deformity and echocardiographic scores of >8 should not be excluded a priori from consideration for percutaneous mitral valvuloplasty. There is no absolute contraindication to percutaneous mitral valvuloplasty in patients with higher echocardiographic scores, but patients with echocardiographic scores of >8 require an individualized approach. The duration of palliation in such patients may be less than that in patients with ideal valve morphology, and the acute procedure success rate is lower. When valve deformity is associated with other clear indications for open heart surgery, the decision is relatively simple. This patient group includes patients with associated significant aortic stenosis or insufficiency, multivessel coronary artery disease, or severe tricuspid regurgitation in need of repair. When none of these indications are present or clear, percutaneous commissurotomy in patients with significant valve deformity can be a successful palliative therapy. This is an especially useful strategy in patients with borderline aortic insufficiency or stenosis, in whom a waiting period after mitral commissurotomy may allow for a more timely decision for double-valve replacement at a later date.
Technique
Several basic techniques of percutaneous mitral valvuloplasty (PMV) are in use. Retrograde transarterial techniques, used alone or in combination with antegrade (trans-septal puncture) techniques, have been used in some centers for singleand double-balloon PMV.
17 They offer the advantage of not requiring trans-septal puncture or using only minimal dilatation of the intra-atrial septum. Disadvantages of these techniques include the risk of arterial injury because of the larger balloons used. In addition, the procedures can be technically difficult and time-consuming.
The most commonly used approaches are transvenous antegrade (i.e., trans-septal) techniques, using either a double-balloon or the Inoue balloon system.
2,
3 The Inoue balloon is the only device approved specifically for percutaneous mitral valvuloplasty in the United States and is the most commonly used device worldwide. Alternatively, a double-balloon technique can be used with two balloons advanced over separate guidewires from the femoral vein to the left atrium, across the mitral valve into the left ventricle.
18 The two balloons are then inflated simultaneously across the mitral valve.
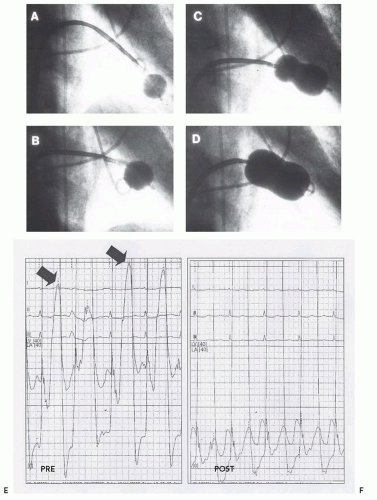
Figure 33.2 illustrates the two-balloon technique. In this patient, the mitral valve was first dilated with a single balloon,
after which double balloons were used to achieve the desired hemodynamic result. When properly performed, the doubleballoon technique results in excellent improvement in mitral valve area.
19,
20 Multiple studies have shown no significant difference in hemodynamic results (mitral valve gradient or mitral valve area) postprocedure between the double-balloon technique and the Inoue balloon system.
21An adaptation of the double-balloon technique uses a monorail approach to deliver two balloons across the mitral valve over a single guidewire.
22 The first valvuloplasty balloon with a short monorail segment is passed over the wire across the mitral valve, followed by a second conventional balloon that is then passed over the wire until it is parallel with the first balloon. There are no substantial differences in the mechanism of delivery of force by two balloons using this approach when compared with the conventional double-wire, double-balloon technique.
In the early surgical era of closed heart mitral commissurotomy, a metallic dilator, or commissurotome, was used via a left ventricular apical incision. Cribier et al.
23 adapted this established surgical technique for percutaneous use. A 19F metallic commissurotome can be passed across the interatrial septum over a guidewire and used to accomplish mitral commissurotomy. There has been some evidence that bicommissural splitting can be accomplished more frequently with the metal commissurotome. Randomized comparisons of the Inoue balloon and metallic commissurotome have not demonstrated significant differences in long-term outcome.
24 This device was never available in the United Sates and is no longer manufactured.
However, the Inoue balloon technique is faster and less cumbersome, and generally requires less fluoroscopy time than these other approaches.
25 The Inoue balloon allows simple progressive upsizing of the balloon without withdrawing the balloon from the left atrium—an important advantage if larger balloon sizes are needed. The Inoue balloon system may, however, result in a slightly higher incidence of mitral regurgitation.
26
Inoue Balloon Technique
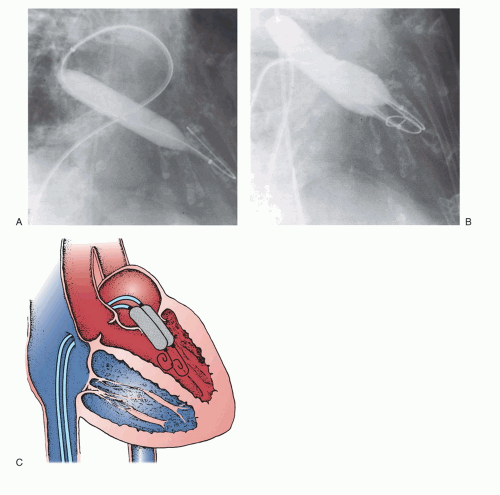
All antegrade approaches begin with the crucial first step of successful trans-septal catheterization. This technique, which is described in
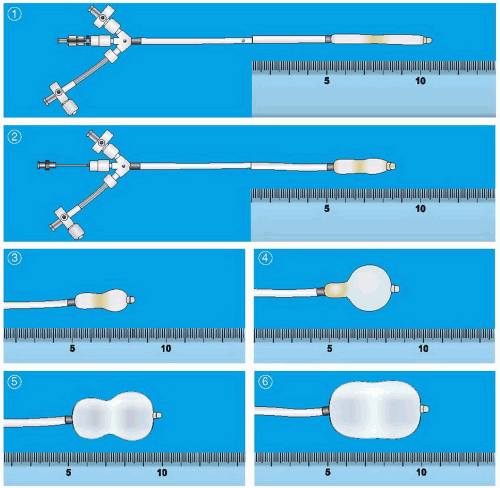
Chapter 6, not only requires successful access to the left atrium, but must also be performed through the appropriate part of the atrial septum to allow easy access to the mitral valve. After successful placement of a Mullins-type dilator and sheath into the left atrium and confirmation of its position by a hand injection of contrast, the patient is anticoagulated with heparin. Baseline hemodynamic measurements are then recorded, confirming the appropriateness of the degree of mitral stenosis for PMV. Subsequently, a special solid-core coiled 0.025-inch guidewire is introduced into the left atrium, and the Mullins sheath dilator system is removed. The femoral vein and interatrial septum are then dilated with a long 14F dilator over the coiled guidewire within the left atrium. The previously prepared, tested, and now slenderized Inoue balloon is then introduced over the guidewire into the left atrium. The Inoue balloon (
Figure 33.3) is made of nylon and rubber micromesh. Owing to the variable elasticity along its length, the balloon inflates in three distinct stages as illustrated in
Figure 33.3. This allows for stable positioning of the balloon catheter across the mitral valve, as described below.
After the slenderized balloon has been positioned within the left atrium, the stretching tube is removed, and a preshaped “J” stylet is introduced into the Inoue balloon. The distal portion of the balloon is inflated slightly to aid in crossing the valve and to prevent intrachordal passage. By maneuvering the balloon catheter while rotating and withdrawing the stylet, the balloon tip can be moved anteriorly and inferiorly toward the mitral orifice. Once the balloon catheter is placed across the mitral orifice, the distal portion of the balloon is inflated more fully and the catheter is pulled back gently to confirm that the inflated distal portion of the balloon is secure across the mitral valve. As further volume is added to the balloon, the proximal end inflates to lock the
valve between the proximal and distal ends of the balloon. Inflation to precalibrated volume then dilates the valve orifice to the corresponding preset size.
Figure 33.4 illustrates the sequential filling and positioning of the Inoue balloon. It is then allowed to deflate passively before it is withdrawn into the left atrium.
The pressure gradient across the mitral valve is measured after each balloon dilatation, and echocardiography may be used to assess the mitral valve area, leaflet mobility, and the degree of mitral regurgitation. If the first inflation has not resulted in a satisfactory increase in the mitral valve area, and the degree of mitral regurgitation has not improved, the balloon is then readvanced across the mitral valve and inflation repeated with the balloon diameter increased by 1 or 2 mm by delivery of slightly more of the precalibrated syringe volume in a stepwise dilatation process, which is repeated until the desired result is achieved.
The Inoue balloon comes in four sizes—24, 26, 28, and 30 mm, referring to the fully inflated maximal balloon diameter. The 24 mm balloon is not available in the United States. However, since actual balloon size is dependent on the volume used for inflation, the actual diameter can be varied over a range from 6 mm less than nominal up to the full rated diameter, as required. We generally estimate the expected maximal inflated balloon diameter using an empirical formula based on the height of the patient (one-tenth the height in centimeter plus 10 mm). It is important to start with a smaller balloon diameter, especially for valves that are very much thickened
or rigid or have moderate amounts of subvalvular disease, to minimize the development of mitral regurgitation, which can develop suddenly with as little as a 1- to 2-mm increase in inflation diameter of the balloon.
The Inoue balloon is fundamentally different from conventional balloons, being volume driven. The balloon is precalibrated so that inflation with volumes labeled on the inflation syringe result in corresponding inflated diameters of the balloon. The pressure that the balloon is inflated to is thus different for different inflation volumes. A smaller maximal-size balloon, such as a 26-mm balloon, when inflated to its maximal size will be at a higher pressure than a balloon that has a larger capacity, such as a 30-mm balloon, inflated to the same diameter of 26 mm. The Inoue balloon has a low-pressure zone encompassing the first two-thirds of its range of inflation. The balloon pressure in this zone typically is approximately 2 or 3 atm. As the balloon is inflated to its last couple of millimeters of diameter with increasing inflation volumes, the balloon pressure rises toward 4 atm. Randomized trials have examined the effects of using balloons in the low-pressure zone as compared with using them in the high-pressure zone.
27,
28 With similar maximal inflated diameters, inflations in the low-pressure zone resulted in less mitral regurgitation than did inflations in the high-pressure zone. Thus, using a 30-mm balloon inflated to a maximum diameter of 28 mm will overall result in causing less mitral regurgitation than will using a maximal nominal 28-mm balloon inflated to 28 mm (in the high-pressure zone).
It is important to assess for increases in mitral regurgitation after each inflation before proceeding to the next inflation diameter. After each balloon inflation, the mean left atrial pressure should be expected to decrease in conjunction with a decrease in the transmitral pressure gradient. When the left atrial pressure remains unchanged both in magnitude and in morphology of the waveform after balloon inflation, it is likely that no progress has been made. If a persistent gradient is present, an additional inflation is warranted. The evaluation becomes more difficult when the left atrial pressure rises after a balloon inflation, or when the waveform changes with an increase in the amplitude of V wave. Decision making is all the more complicated because the presence and size of V waves in the left atrial pressure tracing is often misleading.
A V wave in the left atrial pressure tracing is dynamic. Large V waves are frequently seen in the left atrium in patients with mitral valve disease in the absence of mitral regurgitation reflecting alterations in left atrial compliance; thus, V waves are neither sensitive nor specific for the presence or importance of mitral regurgitation. In percutaneous commissurotomy, it is common to see a V wave diminish during the course of successive successful balloon inflations, reflecting left atrial decompression with improved left atrial compliance
29 (
Figure 33.4). Changes in the V wave must be assessed carefully during percutaneous commissurotomy procedures, but additional information obtained using techniques such as Doppler echocardiography or repeat left ventriculography is necessary to fully interpret these findings.
Following successful mitral valve dilatation, the Inoue balloon is reslenderized by reintroducing first the guidewire and then the stretching tube. The slenderized balloon is subsequently withdrawn from the body over the guidewire. If no sheath has been used, a 10F sheath is inserted into the femoral vein over the guidewire before removal of the wire. It is useful to leave the guidewire across the atrial septal puncture in the left atrium for 3 to 5 minutes after completion of the procedure, while monitoring the systemic arterial pressure. In rare cases, the trans-septal puncture can be made low in the right atrium, and rather than going through the atrial septum, the needle may traverse the right atrial wall and the transverse pericardial sinus, and then enter the inferior border of the left atrium. In this situation, a satisfactory left atrial pressure waveform is still obtained through the tip of the trans-septal needle, and the path of the puncture through the pericardial space is not apparent until the devices are removed at the conclusion of the procedure. If a wire is left in place at the end of the procedure and the blood pressure drops precipitously after a couple of minutes, with the wire in place, a small balloon catheter can be passed back across the puncture site and inflated to stabilize the patient while pericardial centesis is performed and plans for further management are made. This is a rare occurrence, but catastrophic when it does occur. This small step of leaving the wire across the puncture for just a few moments can be lifesaving in such a situation.
Immediate Results
Immediate results of mitral valvuloplasty are assessed by a combination of echo Doppler and hemodynamic measurements. Repeat evaluation of mitral valve area during the procedure by hemodynamic measurements can be performed with reasonable degrees of accuracy in catheterization laboratories equipped with systems featuring computer analysis. Some inaccuracy creeps into the Gorlin formula in the presence of an atrial shunt or mitral regurgitation.
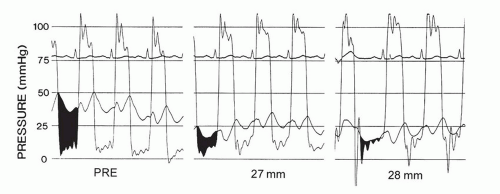
30 Nevertheless, in successful procedures, the mitral valve gradient will be observed to be substantially reduced.
Figure 33.5 illustrates a typical reduction in left atrial pressure and transmitral gradient immediately after balloon mitral valvuloplasty. The mitral valve orifice area will generally be increased to >1 cm
2/m
2 body surface area (
Table 33.2). By echocardiographic assessment in the laboratory, particularly by planimetry of the mitral valve orifice image in the two-dimensional echocardiogram short-axis view, another confirmation of improvement of mitral valve orifice area can be obtained. The accuracy of Doppler measurements during valvuloplasty can be variable, but color Doppler assessment is the method of choice for sequential evaluation of the degree of mitral regurgitation.
37 When Doppler echocardiography is not available in the catheterization lab, serial left ventriculograms can be done to evaluate the degree of mitral regurgitation. The appearance of new mitral regurgitation or an increase of greater than one grade on the 0 to 4 classification of preexisting mitral regurgitation in general signals an end
point of the procedure. In addition, if the mitral valve area has increased to >2 cm
2, or if the mean gradient has been reduced to <5 mmHg without a decrease in cardiac output, the procedure has been completed successfully.
In some cases, a single commissure is split during one of the first balloon inflations. This is often the result of asymmetric commissural fusion or calcification. But splitting of a single commissure often makes it difficult to split the second commissure, since the inflated balloon will be displaced into the already opened side of the valve. This typically results in an adequate rather than an excellent postprocedure valve area. With the Inoue balloon, single commissural splitting typically results in a valve area between 1.6 and 1.8 cm2. When both commissures are split, the valve area is more frequently ≥1.8 cm2 and frequently >2.0 cm2. However, the clinical circumstances and anatomic factors of each patient must be considered carefully in determining the end point of the procedure.
Long-Term Hemodynamic and Clinical Results
Numerous studies have demonstrated the effectiveness of balloon valvuloplasty in increasing mitral valve area.
1,
16 There is a consistent increase in mitral valve area to >1.5 cm
2, a decrease in left atrial pressure, and usually a slight increase in cardiac output. Over time, there is a gradual decrease in pulmonary artery pressure and pulmonary vascular resistance.
38 Long-term follow-up of >4 years have been reported by several single-center registries. These registry analyses have shown quite satisfactory results (
Table 33.3).
39, 40, 41, 42 In a larger multicenter series, the National Heart, Lung, and Blood Institute (NHLBI) Balloon Valvuloplasty Registry reported results in 736 patients older than 18 years who were followed for 4 years.
43 The actuarial survival rates at 1, 2, 3, and 4 years were 93%, 90%, 87%, and 84%, respectively. The event-free survival (freedom from death, mitral valve surgery, or repeat balloon valvuloplasty) at 1, 2, 3, and 4 years was 80%, 71%, 66%, and 62%, respectively. Multivariate predictors of mortality were New York Heart Association (NYHA) functional class IV, echocardiographic mitral valve score of >12, systolic pulmonary artery pressure of >40 mmHg postprocedure, and left ventricular end-diastolic pressure of >15 mmHg. More recently, Bouleti et al. reported long-term follow-up data for up to 20 years in 912 patients who had had successful mitral valvulopasty, defined by a valve area of ≥1.5 cm
2 with mitral regurgitation of ≤2.
44 During a median follow-up of 12 years, 561 patients (62%) were free of reintervention. In the 351 patients (38%) who underwent a reintervention, surgery was performed in 266 patients and repeat balloon valvulopasty in 85 patients. Importantly, cardiovascular survival without surgery was 60 ± 7% at 10 years in the 85 patients who underwent repeat valvulopasty. These data support the concept that percutaneous mitral balloon valvulopasty is an effective therapy for mitral stenosis and that a repeat valvulopasty can allow further postponement of surgery in a significant number of patients.