Introduction
Percutaneous coronary intervention (PCI) has been the most commonly employed coronary revascularization strategy for almost 20 years. In patients with chronic stable angina, the goals of PCI include the relief of angina and ischemia as well as preventing the progression and complications of coronary artery disease (CAD). This chapter describes the principles and evidence basis for coronary intervention technologies and reviews historic and contemporary trials that have compared PCI with medical therapy in stable CAD.
Principles and historic overview
Myocardial ischemia arises principally from an imbalance between myocardial oxygen requirements and supply. Coronary flow reserve (CFR) describes the normal physiologic capacity to increase coronary blood flow (hyperemia) in response to heightened downstream demand through dilation of coronary resistance vessels (principally precapillary arterioles). By definition, hemodynamically significant stenoses in upstream epicardial coronary arteries are those that diminish CFR by preventing physiologic hyperemia, leading to the potential for regional myocardial ischemia. The threshold for ischemia is determined substantially by the severity of the epicardial stenosis, but is also modulated by factors such as collateral flow, myocar-dial viability and blood oxygen content. Successful PCI alleviates the offending epicardial stenosis, thereby preventing ischemia through partial or complete normalization of CFR.
Dotter and Judkins first proposed the concept of transluminal angioplasty in peripheral arteries in 1964.1 Thirteen years later, Gruntzig performed the first balloon angioplasty of a stenotic coronary artery in a conscious human.2,3 The ability of balloon angioplasty to enlarge the vessel lumen and relieve stenosis was originally attributed to compression of the atheromatous plaque.3 However, luminal improvement immediately following balloon or stent-based PCI is actually the result of plaque displacement. Luminal stretching (barotrauma) leads to fracture of the intimal plaque and partial disruption of the media and adventitia, allowing enlargement of the lumen by increasing overall vessel diameter.4 Elastic vessel recoil, post-traumatic neointimal proliferation, and positive (favorable) or negative remodeling of the treated segment determine the final healed result which stabilizes 4 – 6 months following intervention.
Balloon-based percutaneous transluminal coronary angioplasty (PTCA) became widely disseminated in the 1980s. As equipment design and operator experience improved, PTCA was progressively employed in higher risk patients and in more challenging anatomy, including longer lesions, bifurcations, total occlusions and multivessel disease.5 The technique remained limited, however, by dissection and thrombosis of the treated vessel (threatened or actual abrupt closure) requiring emergency CABG in 3 – 5% of patients, and by restenosis of the treated segment in 20 – 30% of patients. A variety of novel devices were developed to solve these problems; however, only coronary stenting has demonstrated improved procedural safety and late clinical outcomes when rigorously compared to balloon angioplasty alone (see below).
Compared to balloon angioplasty alone, modern metallic stents nearly eliminate elastic recoil and compress or seal planes of barotrauma-induced disruption, thereby preventing propagation of dissection and reducing the stimuli for thrombosis that lead to abrupt vessel closure. While metallic stents prevent negative vessel remodeling, they also prevent positive remodeling and have no beneficial effect on neointimal proliferation. Thus bare metal stents have reduced, but by no means eliminated, restenosis. Active antiproliferative drug coatings employed on drug-eluting stents substantially prevent neointimal proliferation and have reduced restenosis rates to below 10%. Bare metal or drug-eluting stents are now used in more than 95% of PCI cases, with procedural success rates greater than 98% and emergency CABG rates less than 1% in most institutions.6 Balloon angioplasty is now most often used to dilate a vessel prior to stent deployment in order to enhance visualization of the target segment, improve stent deliverability, and ensure calcified vessels yield to dilation forces. Balloon angioplasty, often at high pressures (14 – 24 atmospheres), is also employed to further expand the stent and vessel following initial stent deployment. This technique is intended to ensure optimum lumen dimensions and complete apposition of stent struts to the vessel wall, and is associated with a reduced incidence of restenosis and stent thrombosis.6 Stand-alone balloon angioplasty (no stent) is now reserved for specific circumstances: small-caliber vessels (< 2.5 mm), side branch treatment at bifurcations, distal bypass graft anastomotic lesions, select cases of focal in-stent restenosis, and in patients unable to tolerate dual antiplatelet therapy.
Percutaneous coronary intervention technology
PCI systems share three basic components: a guiding catheter that provides stable access to the coronary ostium; a steerable guidewire that is advanced distal to the stenosis and acts as a rail for delivering a variety of therapeutic devices; and a non-elastomeric balloon (with or without a mounted stent) for dilating the physiologically significant epicardial stenosis (Fig. 26.1).
Figure 26.1 (a) Photograph of a guiding catheter that provides stable access to the coronary ostium (1), a non-elastomeric balloon (with a mounted stent) (2) for dilating physiologically significant epicardial stenosis, and a steerable guidewire (3) that is advanced distal to the stenosis and acts as a rail for delivering a variety of therapeutic devices. (b) Expanded 5.0 × 32 mm bare metal stent.

A number of diagnostic and guiding catheters have been developed for coronary arteriography. To allow passage of balloons and other devices, early thick-walled guiding catheters were constructed from large 10 and 11 F tubes that demanded correspondingly large-caliber arterial sheaths with their attendant bleeding risks. Modern guide catheters allow most cases to be completed through 6 F or smaller vascular access sheaths, thereby reducing bleeding risk and enabling radial or femoral artery access (see below).
Modern guidewires combine atraumatic tip softness with precise torque control for steering, graduated support for device delivery, and improved radiographic visibility. Lubricated hydrophilic coatings are employed on specialty wires for tortuous or critically stenotic segments. Stiffer tip designs with different shaft and taper characteristics are now available for crossing chronic total occlusions (CTOs). The large selection of modern guidewires has improved procedural success in a wide range of challenging anatomic substrates.
The cross-sectional profiles of modern angioplasty balloon catheters and stent delivery systems have also decreased substantially while their flexibility has increased. Together with myriad other design improvements, modern balloon catheters and stent delivery systems can readily be made to pass through marked tortuousity and to access distal arterial segments. These improvements may also reduce trauma, endothelial injury and associated plaque progression in coronary segments proximal to the target lesion, though these benefits have not been clearly demonstrated.
A variety of vascular approaches are available for PCI. The right and left femoral arteries are the most commonly used access sites. Although Sones first introduced a cutdown approach for the brachial artery for diagnostic coronary angiography, this approach has been supplanted by percutaneous radial artery access. Access site selection currently depends on operator and patient preference, anticoagulation status, body habitus, and the presence of significant peripheral vascular disease (PVD).
Small trials suggest a transradial approach is preferred in patients with morbid obesity.7,8 The recent Agostoni et al meta-analysis included 12 randomized trials (n = 3224) comparing the transradial and transfemoral approach.9 The risk of major adverse cardiac events (MACE) was similar for both the radial and femoral approach (odds ratio (OR) 0.92, 95% confidence interval (CI) 0.57-1.48; P = 0.7). Radial access was associated with a significantly lower rate of entry site complications and bleeding (OR 0.20, 95% CI 0.09-0.42; P = 0.0001) but a higher rate of procedural failure (OR 3.30, 95% CI 1.63-6.71; P = 0.001). The multicenter OASIS-7/CURRENT trial is enrolling patients and will help clarify the importance of access site selection in PCI.
Bare metal stents
Balloon-expandable metallic stents were the first advance to reduce restenosis, reduce acute complications and provide superior medium-term clinical outcomes when compared to balloon angioplasty alone.
BENESTENT-1 and STRESS were early pivotal trials comparing bare metal stents (BMS) with balloon angioplasty10,11 in simple, short coronary stenoses. Elective Palmaz-Schatz stenting was compared to balloon angioplasty and the results provided the evidence base for FDA approval in 1994 for the prevention of restenosis in de novo lesions. Both studies showed improved initial angiographic results using quantitative coronary angiography (QCA), larger postprocedural minimal luminal diameters (MLD), fewer residual dissections, and decreased clinical and angiographic restenosis at six months. In BENESTENT-1 (n = 520), the mean (± SD) MLD immediately after the procedure were 2.48 ± 0.39 mm in the stent group and 2.05 ± 0.33 mm in the angioplasty group; at follow-up, the diameters were 1.82 ± 0.64 mm in the stent group and 1.73 ± 0.55 mm in the angioplasty group (P = 0.09), which correspond to rates of restenosis (diameter of stenosis (DS) > 50%) of 22% and 32%, respectively (P = 0.02). In STRESS (n = 410), the mean (± SD) MLD immediately after the procedure were 2.49 ± 0.43 in the stent group and 1.99 ± 0.47mm in the angioplasty group (P < 0.001). At six months, the patients with stented lesions continued to have a larger luminal diameter (1.74 ± 0.60 vs 1.56 ± 0.65mm, P = 0.007) and a lower rate of restenosis (DS 31.6% vs 42.1%, P = 0.046). TOSCA-1 was the largest stent versus balloon trial in complex coronary stenoses (non-acute occlusions).12 Stenting again resulted in larger mean six-month MLD (1.48 versus 1.23 mm, P < 0.01) and a reduced binary angiographic restenosis rate (55% versus 70%, P < 0.01).
Bare metal stents were initially limited by stiffness, modest radial strength and inconsistencies in paving which restricted their utility in complex anatomy. Numerous design iterations by stent manufacturers lead to second-, third-and fourth-generation stents that incorporated substantially improved stent flexibility (both before and after stent deployment), superior radial strength and paving, thinner struts, enhanced radiographic visibility, and innovative metallurgy. However, newer trials employed non-inferiority designs to compare different types of bare metal stents, making it difficult to confirm or quantify the effects of improved stent designs.
Two meta-analyses have been published comparing stenting with balloon angioplasty. Brophy et al identified a total of 29 trials involving 9918 patients (Figs 26.2, 26.3).13 No overall difference between strategies of routine coronary stenting versus routine balloon angioplasty was observed for death or myocardial infarction outcomes (odds ratio 0.90, 95% CI 0.72 – 1.11) or the need for coronary artery bypass surgery (odds ratio 1.01, CI 0.79 –1.31). However, nearly all trials allowed or encouraged early cross-over to stenting for patients experiencing balloon angioplasty failure due to marked recoil, dissection and threatened or actual abrupt closure, a design feature that almost certainly obscured from the primary intention-to-treat analyses an important safety advantage provided by stents. Coronary stenting did reduce the rate of restenosis by half (odds ratio 0.52, CI 0.37 – 0.69) and the need for repeated balloon angioplasty by 40% (odds ratio 0.59, CI 0.50–0.68]). Al Suwaidi et al performed a separate meta-analysis of 23 trials and reached similar conclusions.14
Figure 26.2 Meta-analysis of stents versus conventional balloon angioplasty on rate of death or MI, angiographic restenosis, and repeat PTCA. MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty. (From Brophy et al.13)
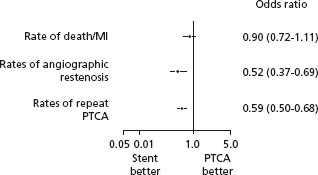
Figure 26.3 Evaluation of the Effects of routine stenting versus conventional PTCA on death and reinfarction. (a) Effects on death; (b) effects on reinfarction. AS, Angioplasty or Stent; BENESTENT, Belgian Netherlands Stent Study; BESMART, Bestent in Small Arteries; BOSS, Balloon Optimization versus Stent Study; CADILLAC, Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications; DESTINI, Doppler Endpoint Stenting International Investigation; EPISTENT, Evaluation of Platelet IIb/IIIa Inhibitor for Stenting; FRESCO, Florence Randomized Elective Stenting in Acute Coronary Occlusions; FROST, French Optimal Stenting Trial; GRAMI, Gianturco-Roubin in Acute Myocardial Infarction; ISAR-SMART, Intracoronary Stenting or Angioplasty for Restenosis Reduction in Small Arteries; MAJIC, Mayo-Japan Investigation for Chronic Total Occlusion; OCBAS, Optimal Coronary Balloon Angioplasty With Provisional Stenting Versus Primary Stent; OPUS, Optimum Percutaneous Transluminal Coronary Angioplasty Compared With Routine Stent Strategy; OR, odds ratio; PASTA, Primary Angioplasty Versus Stent Implantation in Acute Myocardial Infarction; SARECCO, Stent or Angioplasty after Recanalization of Chronic Coronary Occlusions; SISA, Stenting in Small Arteries; SPACTO, Stent versus Percutaneous Angioplasty in ChronicTotal Occlusion; Stent-PAMI, Stent Primary Angioplasty in MI; STENTUIM, Immediate Coronary Angioplasty with Elective Wiktor Stent Implantation Comparedwith Conventional Balloon Angioplasty in Acute Myocardial Infarction; TOSCA, Total Occlusion Study of Canada; other abbreviations as in Fig. 27.2. (From Brophy et al.13)
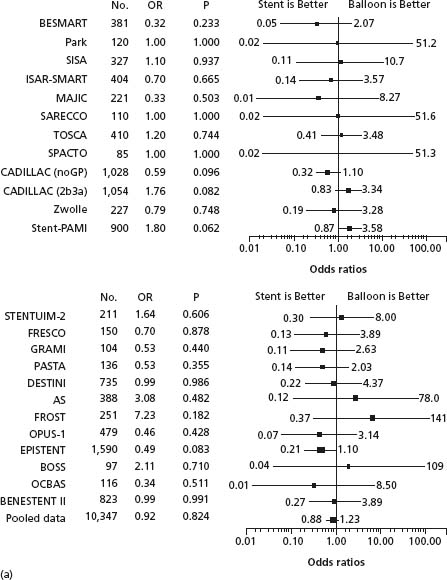
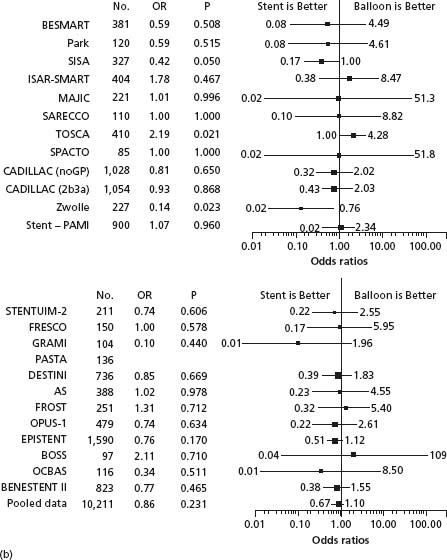
Clinical trials comparing routine stenting to routine balloon angioplasty enrolled anatomically and clinically selected patients. Outcomes in real-world practice including patients with complex stenoses, multivessel disease and a typical burden of co-morbidity were described in a population-based analysis from Canada.15 After adjustment, this analysis showed the adoption of routine bare metal stenting was associated with a 28% reduction in target vessel revascularization and 21% reduction in major adverse cardiac events. Clinical results and outcomes with bare metal stents have continued to improve with the development of more flexible and thin-strut stent designs16 and widespread adoption of modern antithrombotic and antiplatelet therapies administered during and after stent insertion (see below).
Drug-eluting stents (DES)
Although BMS constituted an important advance over balloon angioplasty alone, restenosis due to neointimal overgrowth continues to pose a significant clinical challenge particularly when it is diffuse, proliferative or occlu-sive.17 In a multicenter cross-sectional registry describing the burden of in-stent restenosis in the immediate pre-DES era, approximately 7% of all cardiac catheterization procedures performed involved patients with restenosis of one or more bare metal stents.18 Local delivery of potent antiproliferative drugs via DES has proven an efficacious technology. At the time of writing, four DES device are approved for coronary use in Europe or North America. In order of regulatory approval, these are the Cypher™ (sirolimus eluting, Cordis, Miami Lakes, FL), Taxus™ (paclitaxel eluting, Boston Scientific, Boston, MA), Endeavor™ (zotarolimus eluting, Medtronic, Minneapolis, MN) and XienceV™ (everolimus eluting, Abbott Vascular, Santa Clara, CA). All are composed of a balloon-expandable metallic stent with an adherent polymer that serves as the reservoir for the antiproliferative drug. The drugs elute and diffuse into peri-stent vascular tissue in the weeks following stent deployment where, to a variable degree, they inhibit mitosis of smooth muscle cells that are the primary cellular constituent of restenotic neointimal tissue.
Multiple surrogates have been used to evaluate BMS and DES efficacy. In addition to the quantitative angiographic measures of lumen dimensions and binary restenosis, “ischemia driven” target lesion or target vessel revascular-ization (TLR or TVR) and clinical restenosis are commonly employed clinically relevant endpoints. Angiographic and clinical restenosis rates after BMS implantation typically exceed 20% and 10% respectively, with rates more than doubling in certain patient subsets. Risk factors for restenosis include small reference vessel diameter, long lesion or stent length, and medically treated diabetes mellitus.19 A small minimal stent cross-sectional area on intravascular ultrasound (IVUS) imaging also predicts restenosis (see below).20 In contrast, clinical and angiographic restenosis rates are less than 10% in multiple DES series.21–28
SIRIUS21 and TAXUS IV22 were pivotal trials that demonstrated decreased clinical and angiographic restenosis with drug-eluting versus bare metal stents. Although TLR dramatically decreased, there was again no difference in death or myocardial infarction. In SIRIUS, the mean in-segment (stent plus 5 mm margins) MLD of the sirolimus-coated Bx Velocity stent at 240 days was 2.15 ± 0.61mm vs 1.60 ± 0.72 mm (P < 0.001) for the bare metal stent with a restenosis rate (DS > 50%) of 8.9 versus 36.3% (I < 0.01). In TAXUS IV, the mean in-segment MLD of the paclitaxel-coated Express stent at nine months was 2.03 ± 0.55mm versus 1.68 ± 0.61mm (P < 0.001) for the bare metal stent with a restenosis rate of 7.9 versus 26.6% (P < 0.001).
Two non-inferiority trials have demonstrated similar MLD and restenosis rates with the remaining commercially marketed DES: ENDEAVOR-3 compared zotralimus-elut-ing and sirolimus-eluting stents and SPIRIT-3 compared everolimus-eluting and paclitaxel-eluting stents.
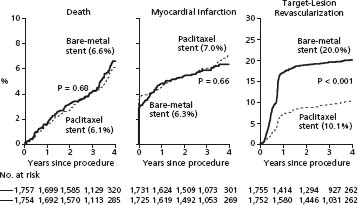
The widespread adoption of DES reduced restenosis and decreased the need for subsequent procedures compared to BMS.23–28b Stone et al performed a pooled analysis of four double-blind trials in which 1748 patients were randomly assigned to receive either sirolimus-eluting stents or BMS and five double-blind trials in which 3513 patients were randomly assigned to receive either paclitaxel-eluting stents or BMS (Figs 26.4, 26.5).23 The four-year rates of stent thrombosis were 1.2% in the sirolimus stent group versus 0.6% in the BMS group (P = 0.20) and 1.3% in the paclitaxel stent group versus 0.9% in the BMS group (P = 0.30). However, after one year, there were five episodes of stent thrombosis in patients with sirolimus-eluting stents versus none in patients with BMS (P = 0.025) and nine episodes in patients with paclitaxel-eluting stents versus two in patients with BMS (P = 0.028). The four-year rates of target lesion revascularization were markedly reduced in both the siro-limus stent and the paclitaxel stent group, as compared with the BMS groups. The rates of death or MI did not differ significantly between the groups that received DES or BMS.
Figure 26.4 Analysis of randomized trials of sirolimus versus BMS on death, MI, and TLR. BMS, bare metal stents; MI, myocardial infarction; TLR, target lesion revascularization. (From Stone et al.23)
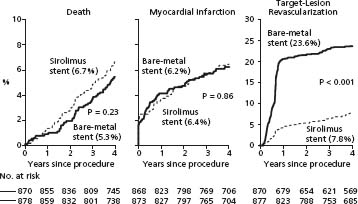
Figure 26.5 Analysis of randomized trials of paclitaxel versus BMS on death, MI, and TLR. Abbreviations as in Fig. 27.4. (From Stone et al.23)
The decreased restenosis rates with DES may come at a price. Although the on-label randomized controlled trials failed to detect an increase in late stent thrombosis with DES, large off-label real-world registries have produced conflicting results. It appears that the increased late stent thrombosis observed with DES may be offset by decreased restenosis-driven myocardial infarctions over time. Large, multicenter trials powered to address these questions are ongoing. Currently, the data as a whole support the following conclusions.29
- DES significantly reduce restenosis compared with BMS.
- Overall stent thrombosis rates are low and occur with both BMS and DES.
- The timing of stent thrombosis appears to vary, with DES more likely to be associated with late and very late stent thrombosis. Cessation of clopidogrel appears to be an important but not universal precipitating factor.
- In the carefully selected and monitored patient populations enrolled in the randomized trials leading to FDA approval, there was no difference in death or MI during follow-up.
- The optimal duration of dual antiplatelet therapy with clopidogrel remains uncertain.
Atherectomy
Plaque volume is a predictor of restenosis.30 The directional atherectomy catheter gained FDA approval in 1990 and allowed cutting and removal of coronary plaque in a controlled (“directional”) manner. It was hypothesized that plaque removal, by avoiding barotrauma and reducing plaque volume, would incite a less vigorous neointimal response and restenosis. However, increased rates of myocardial infarction, death, and major complications without a significant reduction in restenosis were found in adequately powered randomized trials, leading to abandonment of this technology.31–33
Rotational atherectomy (Rotablator™, Boston Scientific, Boston, MA) is a debulking procedure employing a rapidly rotating diamond-tipped abrasive burr (160000 – 180000rpm). The device pulverizes calcified atherosclerotic plaque into tiny particles intended to pass through the microcirculation and be removed by the reticuloendothelial system. Unfortunately, sludging of particles can result in obstruction of the microcirculation with an increased risk of ischemia and non-Q wave myocardial infarction.34–36 Its use is primarily limited, therefore, to removal of superficial calcium that may otherwise prevent lumen and vessel expansion.
Intravascular ultrasound (IVUS)
Since the introduction of IVUS to interventional cardiology in 1988, it has gained acceptance as both a research tool and a clinical modality useful for clarifying epicardial coronary anatomy when the angiographic interpretation is unclear or ambiguous. IVUS can provide not only high spatial resolution images of the vessel lumen and stent, but also detailed and potentially useful information about plaque and arterial wall characteristics.
BOX 26.1 Intravascular imaging ultrasound (IVUS) (modified from 2005 ACC/AHA/SCAI guideline update for percutaneous coronary intervention)
Class IIa indications
IVUS is reasonable for the following:
- Assessment of the adequacy of deployment of coronary stents, including the extent of stent apposition and determination of the minimum luminal diameter within the stent. (Level B)
- Determination of the mechanism of stent restenosis (inadequate expansion versus neointimal proliferation) and to enable selection of appropriate therapy (vascular brachytherapy versus repeat balloon expansion). (Level B)
- Evaluation of coronary obstruction at a location difficult to image by angiography in a patient with a suspected flow-limiting stenosis. (Level C)
- Assessment of a suboptimal angiographic result after PCI. (Level C)
- Establishment of the presence and distribution of coronary calcium in patients for whom adjunctive rotational atherectomy is contemplated. (Level C)
- Determination of plaque location and circumferential distribution for guidance of directional coronary atherectomy. (Level B)
Class IIb indications
IVUS may be considered for the following:
- Determination of the extent of atherosclerosis in patients with characteristic anginal symptoms and a positive functional study with no focal stenoses or mild CAD on angiography. (Level C)
- Preinterventional assessment of lesional characteristics and vessel dimensions as a means to select an optimal revascularization device. (Level C)
- Diagnosis of coronary disease after cardiac transplantation. (Level C)
Class III indications
- IVUS is not recommended when the angiographic diagnosis is clear and no interventional treatment is planned. (Level C)
Despite an extensive literature validating the precision and intraprocedural utility of IVUS, data supporting the ability of this technology to improve clinical outcomes are few. The CLOUT trial evaluated IVUS-guided balloon angioplasty.37 IVUS use resulted in a larger mean MLD, from 1.95 to 2.21 mm (P < 0.0001), and a larger luminal area from 3.2 to 4.5 mm2 (P < 0.0001). Periprocedural complication rates and six-month restenosis rates were nevertheless similar. The SIPS trial (n = 269) compared IVUS-guided versus angiographically guided balloon angioplasty with bailout stenting where appropriate.38 There was a signifi-cant improvement in TLR in the IVUS optimized group at two-year follow-up (17% vs 29%; P p < 0.05).
Subsequent IVUS-guided stent trials have demonstrated that minimum stent area (MSA), as measured by IVUS, is a predictor of late angiographic and clinical restenosis.39 However, the conflicting findings of small IVUS-guided stent optimization trials have been rationalized by the differing angiographic procedural endpoints in the IVUS-guided arms, as well as the disparate adjunctive medical treatment strategies employed (see below).
Recent concerns with late stent thrombosis with DES have fuelled speculation that the mechanism may be related to incomplete stent apposition against the arterial wall.40–42 Using IVUS to guide DES deployment and thereby ensure adequate stent expansion and complete stent – vessel wall apposition has therefore been advocated in anatomically complex target lesions, but large randomized long-term studies that evaluate clinical outcomes after procedures with and without IVUS are needed to confirm this hypothesis. Presently there are no randomized or compelling outcome data that support the contention that routine IVUS during PCI improves clinical results.
Pressure wire
The ratio of the mean coronary artery pressure distal to a stenosis to the mean aortic pressure during maximal vaso-dilation (maximal hyperemia) is called the fractional flow reserve (FFR).43 Comparisons with differing stress testing modalities in patients with stable coronary artery disease revealed that in 45 consecutive patients, the sensitivity of FFR for the identification of reversible ischemia was 88%, the specificity 100%, the positive predictive value 100%, and the negative predictive value 88%. A 0.014 inch pressure sensor-tipped coronary angioplasty guidewire is advanced across a stenosis, and the absolute distal pressure is recorded at rest and at maximal hyperemia induced by intracoronary or intravenous infusion of adenosine. The ischemic threshold value of FFR is < 0.75 and current indications are listed in Box 26.2.
BOX 26.2 Coronary artery pressure and flow: use of fractional flow reserve and coronary vasodilatory reserve (modified from 2005 ACC/AHA/SCAI guideline update for percutaneous coronary intervention)
Class IIa indications
Reasonable to use intracoronary physiologic measurements (IVUS, pressure wire) in the assessment of the effects of intermediate coronary stenoses (30 – 70% luminal narrowing) in patients with anginal symptoms. Coronary pressure or Doppler velocimetry may also be useful as an alternative to performing noninvasive functional testing (e.g. when the functional study is absent or ambiguous) to determine whether an intervention is warranted. (Level B)
Class IIb indications
- Intracoronary physiologic measurements may be considered for the evaluation of the success of PCI in restoring flow reserve and to predict the risk of restenosis. (Level C)
- Preinterventional assessment of lesional characteristics and vessel dimensions as a means to select an optimal revascularization device. (Level C)
- Intracoronary physiologic measurements may be considered for the evaluation of patients with anginal symptoms without an apparent angiographic culprit lesion. (Level C)
Class III indications
- Routine assessment with intracoronary physiologic measurements such as Doppler ultrasound or fractional flow reserve to assess the severity of angiographic disease in patients with a positive, unequivocal non-invasive functional study is not recommended. (Level C)
The appropriateness of intervening on stable coronary artery lesions of intermediate severity and without functional obstruction using pressure wire criteria was assessed in the DEFER study.44 In 325 patients scheduled for PCI of an intermediate stenosis, FFR was measured prior to the planned intervention. If the FFR was ≥ 0.75, patients were randomly assigned to deferral (Defer group, n = 91) or PCI (Perform group, n = 90). If FFR was < 0.75, PCI was performed as planned and these patients were followed up as the reference group (Reference group, n = 144). At five years, event-free survival was not different between the Defer and Perform groups (80% and 73% respectively; P = 0.52), but was significantly worse in the reference group (63%; P = 0.03). The composite rate of cardiac death and acute myocardial infarction in the Defer, Perform, and Reference groups was 3.3%, 7.9%, and 15.7%, respectively (P = 0.21 for Defer vs Perform group; P = 0.003 for the Reference vs both other groups). The risk that a hemodynamically non-significant stenosis (FFR ≥ 0.75) would cause death or myocardial infarction in patients with stable CAD was < 1% per year and was not decreased by stenting.
The efficacy of the pressure wire in multivessel disease was recently assessed in the large multicenter FAME study.45 Before randomization, lesions requiring PCI were identified on the basis of their angiographic appearance. In 1005 randomized patients, those assigned to angiography-guided PCI had drugeluting stents placed in all indicated lesions. Those assigned to FFR-guided PCI underwent stenting of indicated lesions only if the FFR was 0.80 or less. The 1-year rate of death, nonfatal myocardial infarction, and repeat revascularization was 18.3% (91 patients) in the angiography group and 13.2% (67 patients) in the FFR group (P = 0.02). Seventy-eight percent of the patients in the angiography group were free from angina at 1 year versus 81% of the patients in the FFR group (P = 0.20). Like IVUS, routine or selected use of the pressure wire has not yet been demonstrated to improve long-term clinical outcomes.
Adjunctive pharmacology trials
PCI results in de-endothelialization and deep arterial injury that exposes luminal blood to tissue factor and other highly thrombotic molecules. Moreover, the artificial surfaces on catheters, guidewires and implanted metallic stents are themselves thrombogenic. Because thrombosis at the site of PCI can lead to catastrophic ischemic complications, periprocedural administration of antiplatelet and anticoagulant drugs is integral to PCI success and safety. Through intensive and rigorous study, antithrombotic agents and strategies have evolved rapidly over the past three decades.
Aspirin
Aspirin has been universally used in all forms of coronary heart disease for decades, based upon clinical trials performed in a wide variety of clinical settings and presentations.46 Its specific role in PCI for preventing peri-and postprocedural ischemic events was demonstrated in a randomized controlled trial performed in the balloon angioplastyera. The active treatment comprised an oral aspirin/ dipyridamole combination (330/75 mg) given three times daily, beginning 24 hours prior to PCI. Among the 376 randomized patients, there were 16 periprocedural Q-wave myocardial infarctions – 13 in the placebo group and three in the active drug group (6.9% vs 1.6%, P = 0.0113).47 Aspirin has since been standard therapy before and after coronary intervention of all types, and has provided background antiplatelet therapy for all interventional clinical trials testing new antithrombotic regimens, devices and strategies.
The optimal daily dosage of aspirin with a reliable effect in PCI is uncertain. In the USA, 325 mg is common, while in Europe 100 mg or 75 mg is accepted. Higher doses (over 100 mg) are associated with more, predominantly gastrointestinal, side effects but have not been convincingly demonstrated to provide greater long-term protection from thrombotic or ischemic events. Post hoc analysis of the PCI-CURE trial examining patients undergoing BMS placement reported an increased risk of bleeding on long-term higher-dose aspirin (162 – 325 mg) compared to lower dose (75 – 100mg).48 On the other hand, clinically relevant inhibition of platelet aggregation requires 95% blockade of TXA 2 synthesis,49 a level often not reached for 48 hours after initiating daily oral dosages of 75 mg. The OASIS-7/CURRENT trial is presently enrolling patients in a randomized comparison of ASA 81 mg versus 325 mg administered in combination with clopidogrel.
Thienopyridines
Ticlopidine was the first agent in this class to be widely used as an oral adjunct to ASA and heparin during and after PCI. The STARS trial randomized 1653 patients undergoing native coronary artery stenting, including elective procedures for stable CAD, and demonstrated that a combination of aspirin and ticlopidine was superior to an anticoagulation regimen in preventing subacute stent thrombosis at 30 days.50 The benefit was obtained at the expense of increased bleeding complications (mostly access site related), but this trial formed the basis for modern ASA/thienopyridine combination therapy after PCI. Later trials, such as CLASSICS51 and TOPPS,52 demonstrated non-inferiority of clopidogrel vs ticlopidine, in combination with aspirin, for patients undergoing elective stenting. A meta-analysis of randomized and registry comparisons of ticlopidine and clopidogrel post stenting showed clopi-dogrel to be associated with a lower adverse cardiac event rate (2.1% versus 4.0%, P = 0.001) and mortality (0.48% versus 1.09%, P = 0.001) at 30 days.53 These data, together with poor GI tolerance and infrequent but serious blood dyscrasias (neutropenia in ∼ 2% of patients and rare cases of agranulocytosis), have resulted in abandonment of ticlopidine except for those intolerant of clopidogrel.54
The omnipresence of clopidogrel in contemporary PCI trials and guidelines (Box 26.3) has led to its routine use for patients in North America and Europe.55 The duration of clopidogrel therapy was evaluated in the CREDO trial and demonstrated that optimal benefit was achieved by continuing 75 mg daily for 12 months post PCI in stable patients.56 Clopidogrel in combination with aspirin conferred a 3% absolute risk reduction in the combined risk of death, MI or stroke (P = 0.02) when compared to aspirin alone. The timing and dosage of clopidogrel loading prior to PCI for patients not chronically treated appear to influence PCI outcome. Post-hoc
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


