PERCUTANEOUS CORONARY INTERVENTIONS AND OTHER INTERVENTIONAL PROCEDURES
Percutaneous transluminal coronary angioplasty (PTCA) was first introduced by Andreas Gruentzig in 1977 as an alternative to coronary bypass surgery. The concept of percutaneous dilatation of the atherosclerotic peripheral vessels was initially demonstrated by Charles Dotter in 1964 in peripheral vessels where rigid catheters of graduated diameter were used to progressively enlarge the vessel lumen. The development of a small inelastic balloon catheter by Gruentzig allowed expansion of the technique into smaller peripheral and coronary vessels. Initial coronary experience was limited to the small percentage of patients who had single-vessel coronary disease and discrete proximal lesions due to the technical limitations of the equipment. Advances in technology and greater operator experience allowed the procedure to grow rapidly with expanded use in patients with more complex lesions and multivessel disease; by 1990, it was being performed in more than 300,000 patients annually. The addition of atherectomy devices that removed plaques aided in the growth of the procedure, but the introduction of coronary stents in 1994 was one of the major advances in the field. These devices reduced acute complications and reduced by half the significant problem of restenosis (or recurrence of the stenosis). Further reductions in restenosis were achieved by the introduction of drug-eluting stents in 2003. These stents have a polymer coating over the metal stent that is impregnated with antiproliferative agents that slowly release drugs directly into the plaque over a few months. Today, more than 1 million stents are placed in the United States per year and more than 4 million worldwide. Percutaneous coronary intervention (PCI) is the most common revascularization procedure in the United States and is performed nearly twice as often as coronary artery bypass surgery.
The field of interventional cardiology has matured to be recognized as a separate discipline in cardiology that requires specialized training. A dedicated 1-year interventional cardiology fellowship following a 3-year general cardiology fellowship and a separate board certification examination are now required to be certified in interventional cardiology. The discipline has also expanded to include interventions for structural heart disease including treatment of congenital heart disease, and valvular heart disease; it also includes interventions to treat peripheral vascular disease, including atherosclerotic and nonatherosclerotic lesions in the carotid, renal, aortic, and peripheral circulations.
TECHNIQUE
The initial procedure is performed in a similar manner as a diagnostic cardiac catheterization (Chap. 13). As is done with diagnostic catheterization, arterial access is obtained by percutaneous needle puncture into a peripheral artery. Most commonly, the arterial access site is the femoral artery, but radial artery access is gaining favor. To prevent thrombotic complications during the procedure, patients who are anticipated to need an angioplasty are given aspirin (325 mg) and clopidogrel (loading dose of 300–600 mg) before the procedure. During the procedure, anticoagulation is achieved by administration of unfractionated heparin, enoxaparin (a low-molecular-weight heparin), or bivalirudin (a direct thrombin inhibitor). In patients with ST-elevation myocardial infarction, high-risk acute coronary syndrome, or those with a large thrombus in the coronary artery, a glycoprotein IIb/IIIa inhibitor (abciximab, tirofiban, or eptifibatide) may also be given.
Following placement of an introducing sheath, preformed guiding catheters are used to cannulate selectively the origins of the coronary arteries. These catheters have larger internal diameters than diagnostic catheters in order to allow passage of the balloon catheter and wires. Through the guiding catheter, a flexible, steerable guide-wire (diameter 0.4 mm) is negotiated down the coronary artery lumen using fluoroscopic guidance; it is then advanced through the stenosis and into the vessel beyond. This guidewire then serves as a “rail” over which angioplasty balloons, stents, or other therapeutic devices can be advanced to enlarge the narrowed segment of coronary artery. The artery is usually dilated with a balloon catheter and most often a stent is then placed with assessment of the final result by repeat angiography through the guiding catheter. The catheters and introducing sheath are removed and the artery manually held or closed using one of several arterial closure devices to achieve hemostasis. Because PCI is performed under local anesthesia and mild sedation, it requires only a short (1-day) hospitalization that decreases recovery time and hospital expense, as compared to coronary bypass surgery.
The inflated diameter of the angioplasty balloons range in size from 1.5 to 4.0 mm, and balloons are chosen to approximate the “normal” less diseased proximal or distal vessel without stenosis. The major advance introduced by Dr. Gruentzig was the use of inelastic balloons that do not overexpand the vessel beyond their predetermined size despite high pressures up to 10–20 atmospheres.
Angioplasty works by stretching the artery and compressing the plaque into the vessel wall, away from the lumen, enlarging the entire vessel (Figs. 36-1 and 36-2). The procedure rarely results in embolization of atherosclerotic material. Owing to inelastic elements in the plaque, the stretching of the vessel by the balloon results in small localized dissections that can protrude into the lumen and be a nidus for acute thrombus formation. If the dissections are severe, then they can obstruct the lumen or induce a thrombotic occlusion of the artery (acute closure). Stents have largely prevented this complication by holding the dissection flaps up against the vessel wall (Fig. 36-1).
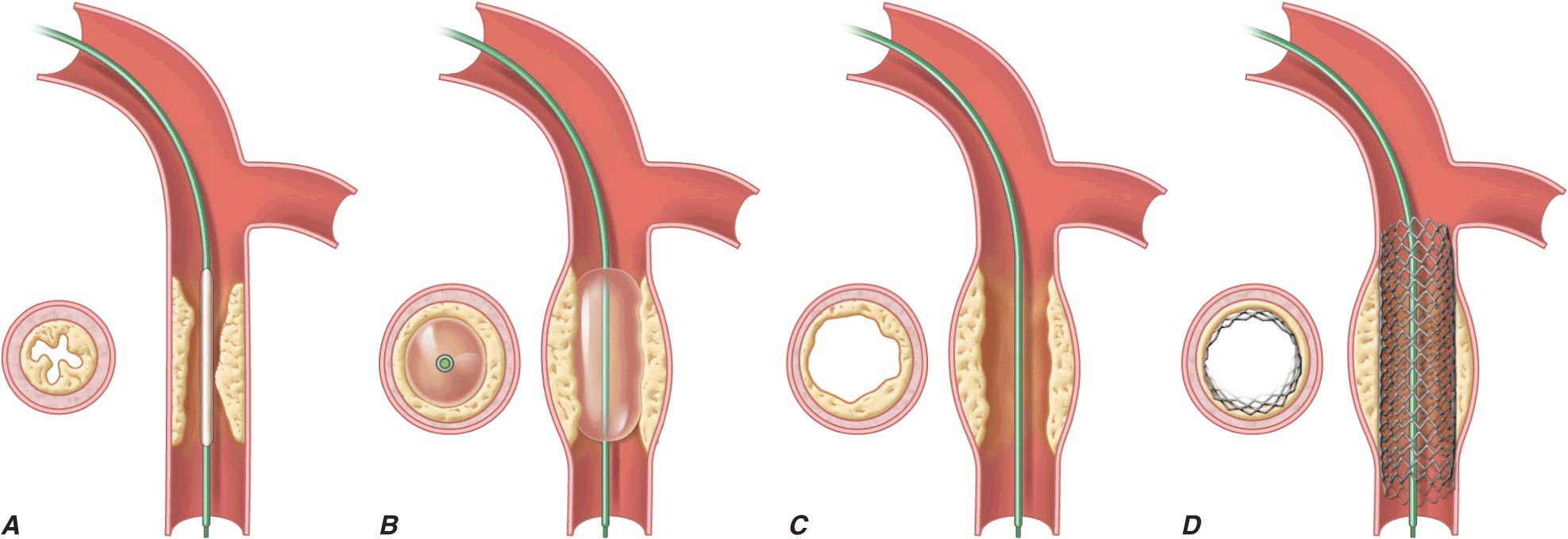
FIGURE 36-1
Schematic diagram of the primary mechanisms of balloon angioplasty and stenting. A. A balloon angioplasty catheter is positioned into the stenosis over a guidewire under fluoroscopic guidance. B. The balloon is inflated temporarily occluding the vessel. C. The lumen is enlarged primarily by stretching the vessel often resulting in small dissections in the neointima. D. A stent mounted on a deflated balloon is placed into the lesion and pressed against the vessel wall with balloon inflation (not shown). The balloon is deflated and removed leaving the stent permanently against the wall acting as a scaffold to hold the dissections against the wall and prevent vessel recoil. (Adapted from EJ Topol: Textbook of Cardiovascular Medicine, 2nd ed. Philadelphia, Lippincott Williams & Wilkins, 2002.)
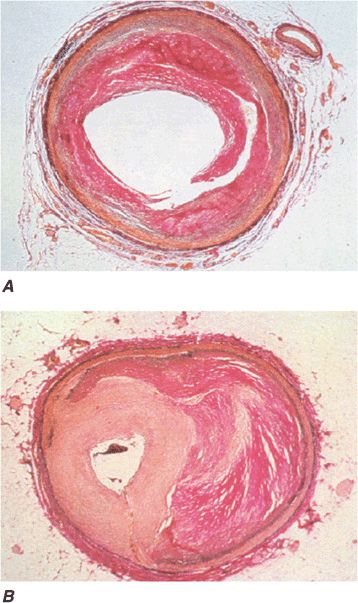
FIGURE 36-2
Pathology of acute effects of balloon angioplasty with intimal dissection and vessel stretching (panel A) (From M Ueda et al: Eur Heart J 12:937, 1991; with permission) and an example of neointimal hyperplasia and restenosis showing renarrowing of the vessel (panel B). (From CE Essed et al: Br Heart J 49:393, 1983; with permission.)
Stents are currently used in more than 90% of coronary angioplasty procedures. Stents are wire meshes (usually made of stainless steel) that are compressed over a deflated angioplasty balloon. When the balloon is inflated, the stent is enlarged to approximate the “normal” vessel lumen. The balloon is then deflated and removed, leaving the stent behind to provide a permanent scaffold in the artery. Owing to the design of the struts, these devices are flexible, allowing their passage through diseased and tortuous coronary vessels. Stents are rigid enough to prevent elastic recoil of the vessel and have dramatically improved the success and safety of the procedure as a result.
Drug-eluting stents were first introduced in 2003. Using a metal stent, an antiproliferative agent is attached to the stent by use of a thin polymer coating. The anti-proliferative drug elutes from the stent over a 1- to 3-month period after implantation. Drug-eluting stents have been shown to reduce clinical restenosis by 50% so that in uncomplicated lesions symptomatic restenosis occurs in 5–12% of patients. Not surprisingly, this led to the rapid acceptance of these devices; currently 50–90% of all stents implanted are drug-eluting. The first-generation devices were coated with either sirolimus or paclitaxel. Sirolimus is an immunosuppressive agent that arrests cell proliferation in the G1 phase. Paclitaxel is an inhibitor of microtubules that can arrest cell division at the M phase in high concentrations, but can have cytostatic G1, antimigratory, and antiinflammatory effects on smooth-muscle cells at lower concentrations. Second-generation drug-eluting stents use newer agents such as everolimus, biolimus, and zotarolimus. These second-generation drug-eluting stents appear to be more effective with fewer complications than the first-generation devices. Preliminary data from long-term follow-up suggests that the second-generation drug-eluting stents have lower rates of stent thrombosis and myocardial infarction than the first-generation drug-eluting stents.
Other interventional devices include atherectomy devices, laser catheters, and thrombectomy catheters. These devices are designed to remove atherosclerotic plaque or thrombus and are used in conjunction with balloon dilatation and stent placement. Rotational atherectomy is the most commonly used adjunctive device for heavily calcified lesions and is modeled after a dentist’s drill, with small round burrs of 1.5–2.5 mm at the tip of a flexible wire shaft. They are passed over the guidewire up to the stenosis and activated to rotate at 180,000 rpm in order to drill away atherosclerotic material. Because the atherosclerotic particles are <25 μm, they pass through the coronary microcirculation and rarely cause problems. The device is particularly useful in heavily calcified plaques that are resistant to balloon dilatation. Another available device is the directional atherectomy catheter. This catheter has a rigid housing at its tip that is open on one side, exposing a sliding rotating cutter. The catheter is placed in the stenosis, and a balloon on the noncutting side of the housing is inflated to push the housing up against the wall of the artery. When the cutter is rotated at 2500 rpm and advanced down the housing, it slices off atherosclerotic plaques into a distal collection chamber, allowing the plaque to be removed from the patient. Given the current advances in stents, neither rotational nor directional atherectomy is as frequently used today as in the past. Other devices include fiberoptic laser catheters that can vaporize atherosclerotic plaques. These are infrequently used today, as well. In acute myocardial infarction, specialized catheters without a balloon are used to aspirate thrombus in order to prevent embolization down the coronary vessel and to improve blood flow before angioplasty and stent placement. Data suggest that manual catheter thrombus aspiration may even reduce mortality rate in primary PCI.
PCI of degenerated saphenous vein graft lesions has been associated with a significant incidence of distal embolization of atherosclerotic material, unlike PCI of native vessel disease. A number of distal protection devices have been shown to significantly reduce embolization and myocardial infarction in this setting. Most devices work by using a collapsible wire mesh at the end of a guidewire that is expanded in the distal vessel before angioplasty. If atherosclerotic debris is dislodged, the basket captures the material, and at the end of the PCI, the basket is pulled into a delivery catheter and the debris safely removed from the patient.
SUCCESS AND COMPLICATIONS
The advances in the technology have greatly improved the success and reduced the complications of the procedure. Currently, a successful procedure (angiographic success), defined as a reduction of the stenosis to less than a 20% diameter narrowing, occurs in 95–99% of patients. The success is dependent upon the coronary anatomy, with lower success rates in patients with tortuous, small, or calcified vessels or chronic total occlusions. Chronic total occlusions have the lowest success rates and their recanalization is usually not attempted unless the occlusion is recent (within 3 months) or there are favorable anatomic features. Improvements in equipment and technique have increased the success rates of recanalization of chronic total occlusions.
Serious complications are rare but include a mortality rate of 0.1–0.3% for elective cases, a large myocardial infarction occurs in less than 3%, and stroke in less than 0.1%. Patients who are elderly (>65 years), undergoing an emergent or urgent procedure, have chronic kidney disease, present with an ST-segment elevation myocardial infarction (STEMI), or are in shock have a significantly higher risk. Scoring systems can help to estimate the risk of the procedure, although no perfect scoring system has yet been developed.
Myocardial infarction during PCI can occur for multiple reasons including an acute occluding thrombus, severe coronary dissection, embolization of thrombus or atherosclerotic material, or closure of a side branch vessel at the site of angioplasty. Most myocardial infarctions are small and only detected by a rise in the creatinine phosphokinase (CPK) or troponin level after the procedure. Only those with significant enzyme elevations (more than three times the upper limit of normal) are associated with a less favorable long-term outcome. Coronary stents have largely prevented coronary dissections due to the scaffolding effect of the stent. Metallic stents are also prone to thrombotic occlusion (1–3%), either acute (<24 h) or subacute (1–30 days), which can be ameliorated by greater attention to full initial stent deployment and the use of dual antiplatelet therapy (aspirin, plus a platelet P2Y12-receptor blocker [clopidogrel or prasugrel]). Late (30 days–1 year) and very late stent thromboses (>1 year) occur very infrequently with stents but are slightly more common with drug-eluting stents, necessitating dual antiplatelet therapy with these stents for up to 1 year or longer. Premature discontinuation of dual antiplatelet therapy particularly in the first month after implantation is associated with a significantly increased risk for stent thrombosis (three- to ninefold greater). Stent thrombosis results in death in 10–20% and a myocardial infarction in 30–70% of patients. Elective surgery that requires discontinuation of antiplatelet therapy after drug-eluting stent implantation should be postponed until after 6 months and preferably after 1 year, if at all possible.
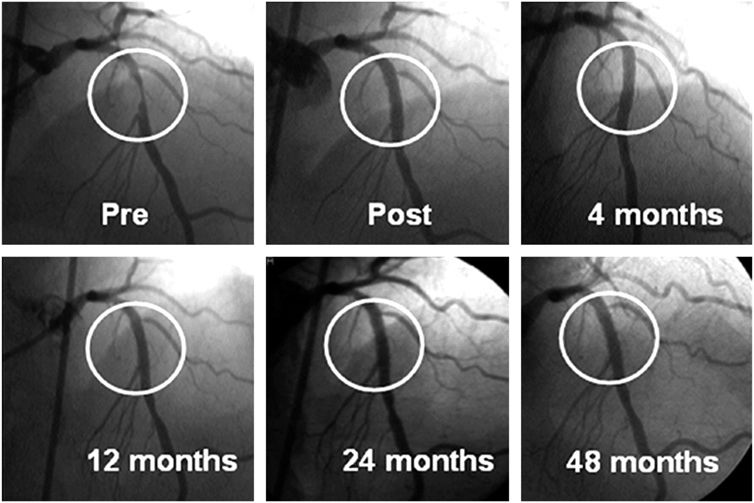
Restenosis, or renarrowing of the dilated coronary stenosis, is the most common complication of angioplasty and occurs in 20–50% of patients with balloon angioplasty alone, 10–30% of patients with bare metal stents, and in 5–15% of patients with drug-eluting stents. The fact that stent placement provides a larger acute luminal area than balloon angioplasty alone reduces the incidence of subsequent restenosis. Drug-eluting stents further reduce restenosis through a reduction in excessive neointimal growth over the stent. If restenosis does not occur, the long-term outcome is excellent (Fig. 36-3). Clinical restenosis is recognized by recurrence of angina or symptoms within 9 months of the procedure. Most commonly, patients with clinical restenosis present with worsening angina (60– 70%), but patients can present with non-ST-elevation myocardial infarction (10%) or ST-elevation myocardial infarction (5%) as well. Clinical restenosis requires confirmation of a significant stenosis at the site of the prior PCI, with repeat PCI or coronary artery bypass grafting (CABG). This is termed target lesion revascularization (TLR) or target vessel revascularization (TVR). By angiography, the incidence of restenosis is significantly higher than clinical restenosis (TLR or TVR) because many patients have mild restenosis that does not result in a recurrence of symptoms. The management of clinical restenosis is usually to repeat the PCI with balloon dilatation and, placement of a bare metal or a drug-eluting stent. Rarely, intracoronary brachytherapy using beta radiation is used. Once a patient has had restenosis, the risk of a second restenosis is further increased. The risk factors for restenosis are diabetes, long lesions, small-diameter vessels, and suboptimal initial PCI result.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree