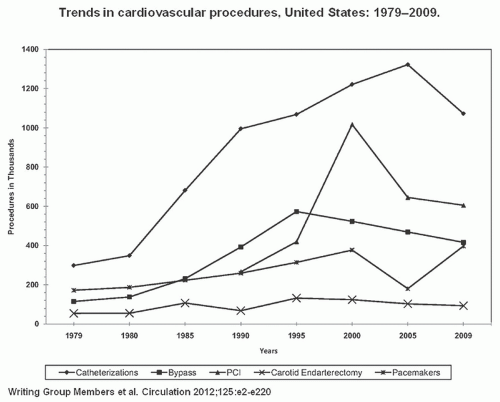
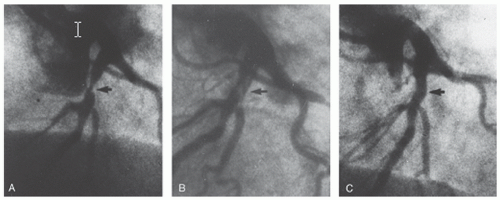
of the National Heart, Lung, and Blood Institute (NHLBI) which had enrolled 3,000 cases by 1981. Over time, progressive improvements in equipment and technique have produced dramatic growth in PTCA and transformed it into the dominant form of coronary revascularization (Figure 28.1). In 2009, approximately 596,000 PCI (in-patient) procedures were performed in the United States5; also it is one of the most common procedures used worldwide.
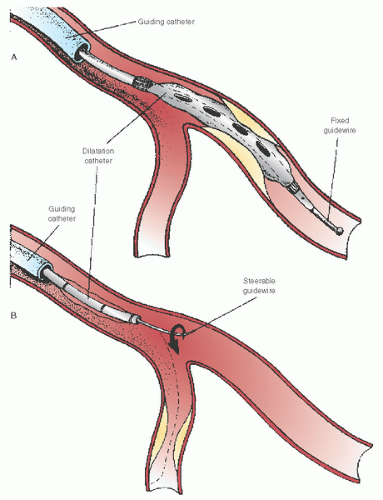
catheter back to avoid its migration into an even deeper position as the device is withdrawn. In this sense, the ability to actively use the guiding catheter constitutes one of the important skills required for effective management of the overall angioplasty equipment system.
coronary circulation to a variety of interventional devices. The basic guidewire consists of a solid core (stainless steel or superelastic nitinol) that is ground to a progressive taper in its distal portion. This taper helps retain torque control when the wire is steered around the series of bends located in the guiding catheter and proximal coronary anatomy and allows the stiffer proximal portions of the wire to follow the soft tip into side branches. This core is generally covered by a spring
coil, and a coating (e.g., Teflon, Silicone) is generally applied to the body of the wire. Radiopaque platinum is often applied to the distal 3 to 25 cm. A family of hydrophilic polymer covered tip guidewires are also available to aid in crossing vessels with extreme tortuosity, calcification, side branches through stent struts, and total occlusion. It must be remembered that hydrophilic wires allow reduced tactile feel and are more likely to cause dissections or perforations.
catheter shaft. This allows the wire to be advanced across the lesion while the balloon catheter remains in the guiding catheter, but does not generally offer sufficient length for exchange of one “over-the-wire” (OTW) device for another. Most guidewires are therefore also available in a 300 cm exchange length, or are extendable to that length by attachment of an extension. Such wires can be passed through the guiding catheter and across the target lesion and remain in place as a series of OTW devices (balloons, rotational atherectomy burrs, stents) are delivered or removed without the need for recrossing the lesion.8 OTW devices have largely been replaced by rapid-exchange (Rx) or monorail balloon catheters and stent delivery systems compatible with shorter guidewires.
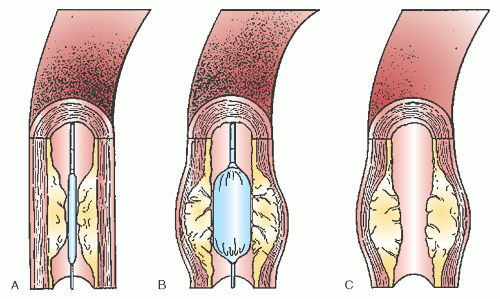
the pressure at which the balloon reaches its specified (nominal) diameter and how much that diameter increases as the balloon is inflated to even higher pressures. More compliant balloon materials tend to reach their nominal diameter at 6 atm and then grow by ≤20% above their nominal size (i.e., a 3.0 mm balloon growing to 3.5 mm) at 10 atm. Semicompliant balloon materials such as high-density polyethylene or nylon grow by <10% over this pressure range, whereas truly noncompliant balloon materials such as PET can retain their defined diameter up to 20 atm to allow dilatation of calcific stenoses or full expansion of coronary stents (Figure 28.4).
giving little incentive for resterilization and reuse, with the risk of infection, prolonged procedure time, and device failures with resterilized products.13,14
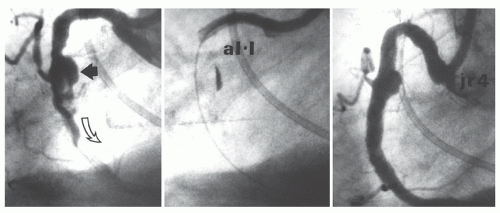
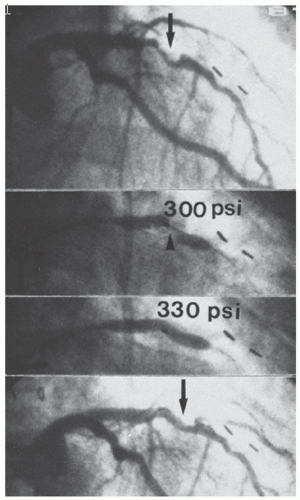 Figure 28.4 Successful dilatation of a rigid calcific lesion (arrows). This rigid lesion (top) in the midleft anterior descending coronary artery of a postbypass patient (note surgical clips) resisted dilatation at 300 lb/in2 (20 atm), but yielded to an inflation pressure of 330 lb/in2 (22 atm; middle two views) with reduction in the stenosis (bottom). Such pressures are obtainable only with high-pressure noncompliant balloons. In current practice, such “nondilatable” lesions would most appropriately be treated by rotational atherectomy (see Chapter 29). |
the only interventions demonstrated to reduce the risk of contrast-induced acute kidney injury (see Chapter 4). It is most important to do so in patients with creatinine clearance of <60 mL/minute. There is now good evidence demonstrating that administration of N-acetylcysteine is not beneficial.
and then steered across the target lesion. The guidewire is advanced across the lesion with the aid of puffs of contrast material through the guiding catheter as the vessel is imaged fluoroscopically in a projection that shows the desired path free of foreshortening or overlapping side branches. Once the position of the wire tip in the distal vasculature has been confirmed by contrast angiography, the desired angioplasty balloon or other device is selected.
PCI. Physiologic assessment can also be done using Doppler flow-measuring guidewires to assess the coronary flow reserve (CFR) as an index of baseline lesion significance and a confirmation of adequate dilation. However, this technique is no longer used in PCI owing to the superiority of FFR as index of stenosis severity, which unlike CFR, is generally not impacted by the presence of microvascular dysfunction. Alternatively, intravascular ultrasound (IVUS; see Chapter 25) or optical coherence tomography (OCT) can more accurately measure lumen diameter and cross-sectional area after dilation, and can detect vessel dissection or hematoma more accurately. Although IVUS has provided important mechanistic insights into balloon angioplasty, it is not used in more than 5% to 10% of routine clinical cases because of the added procedural time and expense. In most laboratories, the postdilation angiogram thus remains the gold standard to assess whether or not an adequate result has been obtained.
of symptoms between 2 and 6 months suggests restenosis of the dilated segment. (Clinically significant restenosis has been reduced markedly from 30% with PTCA to 15% with baremetal stenting and to <5% with drug-eluting stenting.) When symptoms recur 1 or more years after successful angioplasty, it generally suggests progression of disease at another site.44
Table 28.1 Recommended Duration of Dual Antiplatelet Therapy Following Stent Implantation | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
and uniform lumen by angiography or IVUS, with less chance of acute or delayed closure.
elevation, or the no-reflow phenomenon in which there is dramatic reduction in antegrade flow with manifestations of severe ischemia (chest pain and ST-segment elevation), in the absence of epicardial vessel stenosis, dissection, or macroembolic cutoff.55 No-reflow can usually be improved by distal intracoronary injection of an arterial vasodilator (adenosine 12-60 µg; nitroprusside 100 µg; verapamil 100 µg; diltiazem 250 µg; nicardipine 200 µg—but not nitroglycerin, which is more of an epicardial than arteriolar vasodilator). But such treatment does not prevent periprocedural myocardial infarction. In contrast, the use of a distal embolic protection system in vein graft interventions (see Chapter 29) recovers atheroembolic debris and reduces the incidence of these complications by nearly half. The SAFER trial of vein graft stenting thus showed that such enzyme elevations occurred in 17% of lesions, with evidence of no-reflow in 8% of lesions, which were reduced to 9.7% and 3.3%, respectively, through the use of distal embolic protection.56 Similar benefits have now been seen with distal embolic filter devices,57 and in other vascular beds (carotid). However, they have not been shown to improve outcomes in native coronary arteries, but are selectively used by some interventionists in the presence of a large thrombus burden at the site of the culprit lesion.58
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree