Chapter 41 Paroxysmal Supraventricular Tachycardia and Pre-excitation Syndromes
Paroxysmal supraventricular tachycardia (PSVT) has been a well-recognized clinical syndrome and an electrocardiographically defined arrhythmia since the early days of electrocardiography. The clinical syndrome was defined in European literature in the nineteenth century. In 1867, Cotton reported on an “unusually rapid action of the heart,” and was followed by further observations by French and German scientists.1,2 Variously referred to first as Bouveret’s syndrome and later as paroxysmal atrial tachycardia, it was described classically as “a fully unprovoked tachycardia attack, lasting a few seconds or several days, in patients who as a rule have otherwise healthy hearts.”1 It has been electrocardiographically defined in the Tenth Bethesda Conference on Optimal Electrocardiography as “a tachycardia usually characterized by an atrial rate of 140 to 240 beats/min and by an abrupt onset and termination. It may or may not be associated with intact atrioventricular conduction. Specific electrophysiological studies may elicit specific mechanisms such as retrograde and anterograde pathways and sites of re-entry.”3
Epidemiology
The epidemiology of PSVT has not been widely investigated in modern times. Early electrocardiographic reports were useful in arrhythmia detection in patients presenting with sustained palpitations and persistent episodes of supraventricular tachycardias (SVTs) but had little role for this purpose in the large body of patients with brief SVT events terminating before presentation to the physician.4,5 These efforts were supplemented by the advent of ambulatory electrocardiography, which documented a large number of cardiac arrhythmias in asymptomatic patients. Conversely, it confirmed that many symptoms experienced by patients with or without heart disease that are suggestive of tachyarrhythmias occur when no arrhythmias or simply premature beats are documented on monitoring. Epidemiologic data are extensively colored by the selection criteria and the electrocardiographic documentation mode used in the study. Brodsky et al reported that ambulatory electrocardiographic recordings in 50 male medical students detected atrial premature beats in 56%, but only one had more than 100 beats in 24 hours.6 The limited period of observation precludes objective assessment of development of SVT events in these subjects. Thus Hinkle et al, in a study of 301 men with a median age of 56 years, detected various supraventricular arrhythmias in 76% of these individuals.7 However, coronary disease was present in 20% of these patients. Clark et al studied an apparently normal population ranging from 16 to 65 years old and noted a low incidence of supraventricular arrhythmias.8,9 In contrast, recent longitudinal studies with telemetric monitoring and even implanted cardiac pacemakers document a very high incidence of asymptomatic and symptomatic atrial arrhythmias, particularly atrial fibrillation (AF) in patients with bradyarrhythmias.10 Thus, it would be appropriate to infer that the true incidence of PSVT in the general population remains unknown because of its evanescent nature and limited methods of detection. It would also be appropriate to surmise that the incidence may be higher than generally believed over a long observation period. Atrial arrhythmias also increase with age.11 In the Cardiovascular Health Study, short runs of PSVT occurred in 50% of men and 48% of women, doubling in prevalence in octogenarians. PSVT has been shown to occur in 28% of nursing home residents.11
More data are available for pre-excitation syndromes, particularly Wolff-Parkinson-White (WPW) syndrome. Electrocardiographic studies of healthy individuals suggest that the incidence of this condition is 3 in 1000 of the general population.12 Early studies suggested that the morbidity and mortality in WPW syndrome with tachyarrhythmias were greater in adults with ventricular fibrillation (VF) occurring in those with AF and antegrade pre-excitation.13 However, Klein et al have reported on the natural history of asymptomatic WPW syndrome; they noted a very low incidence of major morbidity and serious symptomatic arrhythmias, including AF with rapid antegrade conduction and mortality.14
Supraventricular arrhythmias are seen at all ages and are particularly common in infants, children, and young adults. The age-related behavior of these rhythms has also been the subject of epidemiologic study. In a study of infants younger than 1 year, Mantakas et al noted that associated congenital heart disease was present in 35%, and 90% developed SVTs with narrow (35%) or wide QRS (45%) complexes.15 Most patients (90%) improved with growth or remained stable, but patients with congenital heart disease could have refractory arrhythmias. The quality and duration of life in children with WPW syndrome without clinical dysrhythmias have been reported to be normal.
Anatomy and Pathology
Normal Anatomy of the Sinus Node
Tachycardias of sinus node origin have been described. These can present as paroxysmal episodes of SVT or inappropriate sinus tachycardia (IST), which is nonparoxysmal in nature. Postural orthostatic tachycardia syndrome is discussed elsewhere in the text and is associated with sinus tachycardia and orthostasis. Sinoatrial re-entrant tachycardias (SARTs) arise in the region of the sinus node and peri-nodal region. See Chapter 39 for a detailed treatment of the normal anatomy of the sinus node.
Normal Anatomy of the Atrioventricular Junction
To understand the pathologic base for atrioventricular (AV) nodal tachycardia and AV re-entrant tachycardia (AVRT), the normal anatomy of the AV node and its approaches, including the atria and the AV bundle, is briefly reviewed in the following regions of interest: (1) approaches to the AV node including the atrial septum, (2) AV node, and (3) AV node–bundle junction.16–27
Approaches to the Atrioventricular Node
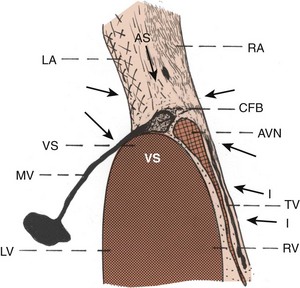
Approaches to the AV node include the atrial myocardium located in the anterior, superior, midseptal, and inferior regions as they converge to the AV node. The approaches beneath the coronary sinus area may be called the posterior or inferior approaches. In addition, approaches also originate from the tricuspid valve. Left-sided approaches include those from the left atrial myocardium and the mitral valve. Superior approaches include the pectinate muscles as they merge from the superior, lateral, and posterior walls of the right atrium toward the AV nodal area, atrial septum, and Todaro’s tendon. Approaches to the AV node are formed by different types of myocardial fibers coming from different directions as they merge toward the AV nodal area (Figure 41-1). Histologically, in general, the cells are relatively loosely arranged with lighter-staining, smaller cells. The size and shape of the myocardial fibers vary considerably from one approach to the other in the vicinity of the node. In general, there is increase in the elastic collagen connective tissue intermingling with the cells, fat, and a large amount of nerve fibers. At the electron microscopic level, the mitochondria and myofibrils of the atrial myocardial cells are not as well organized as the ventricular cells, and some of them do not have a transverse tubular system.16
Significance of the Size Variations of the Approaches to the Atrioventricular Node
Atrial myocardial fibers, including collagen, elastic tissue, and nerve elements in the approaches to the AV nodal area, can vary in size, shape, and direction, suggesting that there may be functional differences in the speed of conduction.16–20 Anatomically, one approach to the AV node may be more dominant than the other. For example, dominant posterior approaches with less prominent superior approaches in humans suggest the possibility of dominant slow pathway conduction. Likewise, dominant superior approaches with practically absent inferior approaches suggest the possibility of the dominance of fast pathway conduction or other alterations in AV nodal conduction. Myocardial fibers in the atria may get entrapped within the central fibrous body and join the AV node. In other instances, approaches to the AV node as well as the AV node may be entrapped within the tricuspid or mitral valve annulus or the base of the aortic valve. During an altered physiologic state, these anomalies may form an anatomic substrate for a re-entry circuit, resulting in various types of supraventricular or junctional arrhythmias.16–18
Atrioventricular Node
The AV node is normally in continuity with its atrial approaches.16–2326 It is a sizable structure and is usually located near the annulus of the septal leaflet of the tricuspid valve. In the adult, it measures approximately 5 to 7 mm in length and 2 to 5 mm in width. The node extends closely beneath the endocardium of the right atrium, adjacent to the septal leaflet of the tricuspid valve, and lies very close to the right ventricular aspect of the ventricular septum and the central fibrous body. The size and shape of the node are not uniform in nature and vary considerably from heart to heart. At the light microscopic level, the AV node consists of a meshwork of cells that are approximately the size of atrial cells but are smaller than ventricular cells. Histologically, the AV node may be divided into three layers: (1) superficial, or subendocardial, (2) intermediate, or midzone, and (3) deep, or innermost.16–18
Superficial or Subendocardial Layer of the Atrioventricular Node
The approaches that come from various directions merge gradually with the superficial or subendocardial part of the AV node. These fibers are loosely arranged with smaller nodelike cells oriented along the atrial cells, some intermingling with the atrial cells, fat, elastic tissue, collagen, and nerve fibers. A distinct increase in fat occurs with normal aging of the heart.16–18
Intermediate or Midlayer of the Atrioventricular Node
AV nodal cells are more or less compact; however, the orientation and arrangement of the cells vary considerably. The collagen and elastic tissue content is less than that seen in the superficial layer with fewer nerve fibers. In the older age group, fat may be present in the intermediate layer of the node.16–18
Deep or Innermost Layer of the Node
AV nodal cells are tightly arranged and may be considered compact. However, the arrangement and orientation of these cells also vary considerably. Some amounts of collagen and elastic tissue are present, though somewhat less than in the intermediate and superficial layers. Fat may be seen intermingling with the nodal cells in patients in the older age group. At the light microscopic level, the nodal fibers vary from the periphery toward the central fibrous body.16–18 At the electron microscopic level, fewer myofibrils and mitochondria are present and are randomly arranged. The cytoplasmic reticulum is poorly developed, and no transverse tubular system exists. It is not known whether the AV node contains more glycogen than the surrounding atrial and ventricular myocardium. The gap junctions are scarce, but desmosomes are frequent. Fascia adherens are more than in the sinoatrial nodal cells but not as frequent as in atrial and ventricular myocardial cells.16–18
Blood Supply and Nerve Supply to the Atrioventricular Node
In approximately 90% of hearts, the AV node is supplied by ramus septi fibrosi, a branch from the right coronary artery reinforced by branches from the anterior descending coronary artery.16–23 Copious nerve cells surround the AV node, especially in the atrial septum adjacent to the AV node and nerve fibers within the node. The exact distribution and destination of the nerves in the AV nodal area in humans are still unknown. However, the rich autonomic innervation of the sinoatrial node and AV nodal areas in the canine heart indicates that the sinoatrial node is particularly responsive to parasympathetic adrenergic regulation, whereas the AV nodal conduction is preferentially sensitive to sympathetic adrenergic regulation.
Variations in Size, Shape, and Location of the Atrioventricular Node
The node lies beneath the septal leaflet of the tricuspid valve close to the right ventricular aspect of the ventricular septum and the central fibrous body. The AV node may be draped over the central fibrous body within the atrial septum, or some of the fibers may be partly within the tricuspid valve annulus and may be partly within the central fibrous body. In some cases, the fibers may be situated toward the left atrial aspect or partly within the mitral valve annulus, or they may be situated close to the base of the aortic valve.16–23 AV node–like cells may also be seen near the tricuspid, mitral, and aortic valve annuli. Some of these nodelike cells may enter the central fibrous body and eventually join the regular posterior AV node. In addition, an accessory AV node may be seen anterosuperiorly in the parietal wall of the right atrium near the annulus of the tricuspid valve.16 Note that not all the AV nodal cells eventually form the AV bundle; some, such as leftover nodal cells, remain and lie adjacent to the central fibrous body or near the valves.
Mahaim Fibers
Conduction fibers from the AV node, AV bundle, and left bundle branch may join the ventricular myocardium. They have been referred to as Mahaim fibers, or paraspecific fibers of Mahaim. They may form the substrate for a unique variety of ventricular pre-excitation. The myocardial fibers resemble the cells of the tissue of origin and gradually take over the characteristics of the ventricular myocardial cells. Mahaim fibers may be present from the AV node to the right, left, or middle part of the ventricular septum.16–23
Accessory Atrioventricular Bypass Pathways—Fibers of Kent
Accessory AV bypass pathways bypassing the AV node are seen in normal infants up to 6 months of age.21 The myocardial cells on the atrial side resemble atrial cells, and those on the ventricular aspect resemble ventricular cells.16–22 In adults, it has been well documented that such pathways cause pre-excitation with varying types of supraventricular arrhythmias.
Atrioventricular Node–Bundle Junction
The AV node penetrates the central fibrous body to become the penetrating part of the AV bundle. The penetrating AV bundle undergoes or assumes different shapes and contours. The orientation of fibers differs significantly. The node-bundle junction is very small, measuring approximately 1 to 1.5 mm in greatest dimension or less in the majority of hearts. The node-bundle junction becomes a part of the AV node and may be considered the most distal part of the AV node or the beginning part of the penetrating AV bundle.16–18 The AV nodal cells that are close to the tricuspid valve and the posterior approaches are first molded to form the penetrating AV bundle. Conversely, the nodal fibers from the anterosuperior aspect are the last to lose their continuity with the superior approaches as they enter the central fibrous body to form the AV bundle. Thus, the formation of the AV bundle within the central fibrous body occurs differentially. The posterior part of the AV node penetrates the central fibrous body earlier than the anterior or superior part. The superior part of the AV node includes the nodal fibers closer to the left atrial side, atrial septal, and right atrial aspects.16–18
Functional Significance of the Node-Bundle Junction
The variation in sizes of the superior and inferior components of the node, as well as the variations in which they form the AV bundle, may provide a substrate for arrhythmias. Since histologically the node-bundle junction has the characteristics of both the AV node and the AV bundle, its functional properties may be intermediate, having characteristics of both the node and the penetrating AV bundle. Likewise, the atrial myocardial approaches from the mitral valve, tricuspid valve, the right ventricular myocardium, and the atrial septum tend to get entrapped within the central fibrous body and may later join the AV node, the node-bundle junction, or both. The normal variations in morphology of the AV junction have the potential for supporting AV nodal arrhythmias.16–18 The variation in the morphology of the AV junctional area also probably predisposes to varying types of physiological phenomena, including dual AV nodal pathways and other junctional arrhythmias.15,16 For example, an anterior AV node in the parietal wall of the atrium or near the tricuspid valve annulus or double AV node may alter its conduction velocity. Dual AV nodal pathways may be normal or abnormal. They may be transient or become permanent.16–18
Accessory Atrioventricular Node and Its Relationship to Pre-excitation and Atrioventricular Junctional Tachycardias
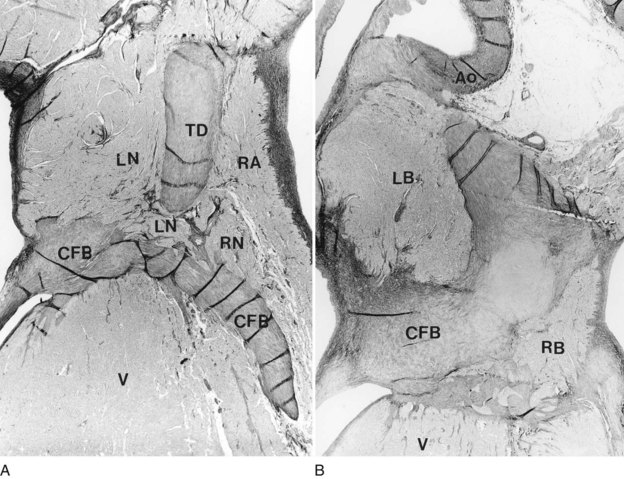
Few pathologic studies document an accessory AV node being responsible for pre-excitation or other arrhythmias originating from the AV junction. Figure 41-2 shows a 5-month-old infant with a history of intractable junctional tachycardia. He died suddenly, and postmortem examination demonstrated a displaced coronary sinus relative to the septal leaflet of the tricuspid valve with a double AV node and double AV bundle (see Figure 41-2). In other instances, the AV node has been located partly within the central fibrous body with a left-sided AV bundle, or the right AV node has been completely interrupted by sutures, and a left-sided AV node was connected to the atrial septum. It was recently documented that an accessory AV node located anterior to the AV junction directly communicated with the right atrium and right ventricle in a curved fashion that produced ventricular pre-excitation and formed the retrograde limb of typical AVRT. In another patient with typical pre-excitation and recurrent AVRT who died suddenly, no conventional anomalous pathways were present on the right side. Instead, an anterior accessory AV node in the right atrium continued as the infundibular right ventricular myocardium.
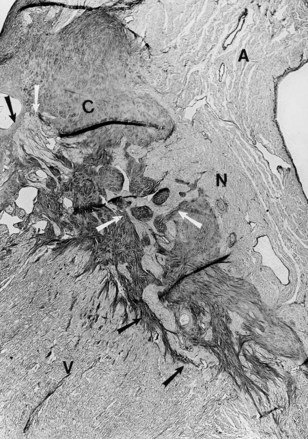
These types of abnormalities of the AV junction are seen in children as well as in adults. Pathologic study in a 13-month-old child with hypertrophic cardiomyopathy and paroxysmal SVTs, consistent with AV reciprocating tachycardia using a concealed posterior accessory pathway, revealed that the central fibrous body was abnormally formed with numerous Mahaim fibers (nodo-ventricular) on both sides of the septum with fibrosis of the left bundle branch (Figure 41-3).
Concepts and Classification
The fundamental concepts underlying recurrent PSVT have been elucidated by decades of experimental and clinical electrophysiological investigations. Early experimental studies of Moe et al and the seminal clinical studies of Durrer, Wellens, Castellanos, Rosen, and Denes, among others, helped define the mechanisms and substrates involved in these arrhythmias.28–31 Extensive pathologic studies, as mentioned earlier, have further enhanced our understanding of the anatomic basis of these arrhythmias. Fundamental to our understanding is the concept that these arrhythmias may be caused by either enhanced automaticity or re-entry. Although the latter may predominate in certain populations and clinical practice, considerable overlap exists in clinical and electrocardiographic features. Automatic arrhythmias may arise from the sinoatrial region and from the working atrial myocardium or the AV junction. Re-entrant rhythms may arise in these structures as well and may also involve accessory AV connections or other variants in the pre-excitation syndrome. Box 41-1 lists the PSVT categories by electrophysiological mechanisms and substrate. A summary of the basic concepts involved in the genesis of these arrhythmias is provided in the following discussion.
Basic Electrophysiology
The anatomic and electrophysiological substrate and physiology of SVTs have been given fresh investigative impetus by the evolution of catheter ablation procedures. Sinus node tachycardias have been simulated in experimental studies using epinephrine injection into the fat pad.32 (Figure 41-4, A).These show evidence of origin either in the sinus node itself or in the superior aspect of the crista terminalis. These have been ablated by chemical or electrical techniques.
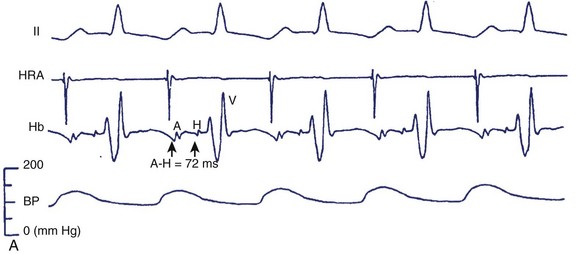
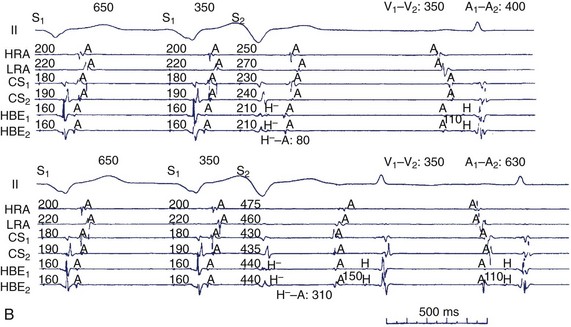
FIGURE 41-4 A, A typical response to the injection of epinephrine into the fat pad. Traces, from top: Electrocardiogram (ECG) lead II; electrograms for the high right atrium (HRA) and bundle of His (Hb) showing atrial (A) His bundle (H) and ventricular activation (V). A, During the control state at a sinus rate of 166 per beats/min the A-H interval measured 72 ms and systolic blood pressure (SBP) was 157 mm Hg. B, After 0.3 mg of epinephrine was injected into the fat pad, the sinus rate increased to 203 beats/min, but the A-H interval and SBP were relatively unchanged (72 ms and 150 mm Hg, respectively). B, Induction of retrograde dual atrioventricular (AV) nodal pathway conduction with ventricular extrastimulation (same patient as in Figure 41-2). H, Retrograde bundle of His potentials. Retrograde atrial activation sequence resulting from retrograde fast AV nodal pathway conduction (A) is first the low septal right atrium (SRA) in the bundle of His electrographic lead (HBE), followed by the proximal coronary sinus (CS1 and CS2), high right atrium (HRA) and lateral right atrium (LRA), whereas that resulting from retrograde slow AV nodal pathway conduction (B) is first CS1 and CS2, followed by low SRA, LRA, and HRA. The morphology of the atrial electrogram changes as a result of the shift from retrograde fast to retrograde slow AV nodal pathway conduction. Ventriculoatrial conduction time (S0A interval) is measured from the stimulus to the onset of atrial electrogram at each recording site and is expressed in milliseconds.
(From Sung RJ, Waxman H, Saksena S, et al: Sequence of retrograde atrial activation in patients with dual atrioventricular nodal pathways, Circulation 64:1053–1060, 1981.)
Atrioventricular Nodal Re-entrant Tachycardias
Pioneering anatomic studies of Tawara and Kent and physiological demonstration of dual AV nodal physiology in dogs by Moe have laid the foundation for our current understanding of this condition.28,33,34 Tawara and others examined the compact AV node, but more recent studies have focused on the connections of the node. Moe et al first suggested the presence of functionally and spatially distinct pathways with fast and slow conduction properties in the canine AV node.30 Subsequently, the cellular electrophysiological properties of the node and its environs were studied (see Chapters 4 and 24). The AN and N regions lie in the triangle of Koch framed by Todaro’s tendon, the tricuspid annulus, and the ostium of the coronary sinus. This anatomic location is critical to the understanding of the technique of catheter ablation of this arrhythmia. The N region is located at the apex of the triangle, where the NH region in the nonbranching part of the AV bundle penetrates the central fibrous body to become the bundle of His. Considerable uncertainty existed in the understanding of the AN region and the atrial connections of the AV node. Truex and Smythe demonstrated atrial extensions of the compact AV node, with a prominent posterior tail that extended to the ostium of the coronary sinus.24 Anderson et al defined superficial, deep, and posterior zones in these connections.35 The superficial zone extended into the anterosuperior part of the node, the posterior zone into the inferoposterior aspect of the compact node, and the deep fibers connected the left atrial septum to the node. Anderson noted that this latter connection joins the distal part of the node, suggesting the possibility of a lesser degree of AV nodal conduction modulation by slow currents and more rapid AV conduction. Definition of these three inputs further refined our clinical techniques of AV nodal modification or ablation. Racker also described discrete superior, medial, and lateral atrio-nodal bundles.36 The medial and lateral bundles converged into a single proximal AV bundle leading into the compact AV node. These two bundles are believed to form, in part or in entirety, the anterior “fast” and posterior “slow” AV nodal physiological pathways.
Moe et al demonstrated the electrophysiological substrate for AV nodal re-entrant tachycardias (AVNRTs) by developing the concept of dual AV nodal pathways as its basis.28 They noted a marked prolongation of AV nodal conduction when a premature atrial extrastimulus was delivered at a critical coupling interval. This was often associated with an echo beat, postulated to be caused by two AV nodal pathways—a β-pathway with a faster conduction and long refractoriness and an α-pathway with shorter refractoriness and slower conduction times. At critical coupling intervals, the premature beat shifts conduction from the β-pathway to the α-pathway and re-engages the β-pathway in a retrograde direction to result in an atrial echo beat. More recent, elegant studies on isolated canine and pig hearts by McGuire, de Bakker, and Janse have shown nodal type action potentials in the cells around both mitral and tricuspid valve rings.37 These cells are separated from atrial cells by a zone of cells with intermediate action potentials. Adenosine reduced the amplitude and upstroke velocity of action potentials in nodal-type cells, but their morphologic characteristics were indistinguishable from those of atrial myocytes. However, they could be distinguished by the absence of connexin43, which was present in the atrium and the ventricle. Posterior AV nodal approaches were dissociated by pacing from the atrium and the AV node, and echo beats used the slow posterior pathway in a retrograde direction and preceded atrial activation. These posterior approaches were not used in fast pathway conduction.
Human Correlates of Experimental Observations
Clinical correlation of these experimental concepts of AV nodal physiology was demonstrated by the early work of Bigger and Goldreyer, as well as by Denes, Rosen, and coworkers.31,38 The critical role of AV nodal conduction delay in the onset of SVT was recognized. Denes et al demonstrated longitudinal dissociation of the AV nodal conduction in patients with recurrent SVT.31 The critical link between anatomic and physiological concepts in humans was provided by a landmark study by Sung et al.39 They demonstrated the posterior input and output of the “slow” AV nodal pathway and the anterior location of the “fast” AV nodal conduction pathway (see Figure 41-4, B). Although submerged in controversy for some time, this observation formed the basis for the development of fast and slow pathway ablation for the cure of AV nodal re-entry. Several years later, this study was directly validated in intraoperative studies in humans. McGuire et al mapped Koch’s triangle to define the earliest atrial activation during AVNRT in patients during cardiac surgery. A zone of slow conduction was found in the triangle in 64% of patients.40 Atrial activation patterns confirmed that the fast pathway was connecting at the apex of the triangle near the AV node–bundle of His junction, whereas the slow pathway did so at the orifice of the coronary sinus near the posterior aspect of the AV node. Two types of AVNRT were distinguished: (1) the common type, or type 1, called the “slow-fast” form, which used the slow pathway for anterograde propagation and the fast pathway for retrograde conduction; and (2) the uncommon type, or type 2, called “fast-slow” AVNRT. Contiguous ablation lesions can affect both pathways, confirming their proximity. The presence of multiple “slow” pathways has also been identified, resulting in a third form of re-entry, called “slow-slow,” or type 3, AVNRT. These different types and pathways support the concept that these arrhythmias are supported by AV nodal tissue, transitional atrio-nodal inputs, and other peri-nodal tissues, which have varying electrophysiological properties and functionally simulate distinct electrical pathways.
Atrioventricular Re-entrant Tachycardias
The classification of pre-excitation syndromes has evolved since the original eponyms were given to Kent, Mahaim, and James fibers. The long-proposed and accepted classification by the European Study Group is shown in Box 41-2. However, limitations of this classification are being recognized, and correlation with the previous eponyms remains occasionally tangential. The major reason for this observation is the recognition that decremental conduction is a property not solely confined to the AV node but also seen with accessory AV connections. Speculation around the embryologic basis of these connections swirls around the original suggestion by Gallagher that these may be displaced AV nodal and specialized conduction system tissues.41 Decremental conduction has been observed in posteroseptal AV connections in the permanent form of junctional reciprocating tachycardia, as well as in atrio-fascicular bypass tracts, which commonly link the parietal atrial myocardium in the right atrium to the right bundle branch at its distal portion and are detected as Mahaim physiology. The tachycardia propagates in an antegrade direction over the atrio-fascicular pathway and in a retrograde direction over the normal AV conduction system and involves the atrium and the ventricle as critical elements in the circuit. This is quite distinct from the nodo-ventricular connections originally described by Mahaim. In this latter instance, the tachycardia has a similar propagation sequence but can exist totally without atrial involvement and has been referred to as a “subjunctional tachycardia.”
Box 41-2 Classification of Pre-excitation Syndromes
Supraventricular Tachycardias Caused by Enhanced Automaticity
Nonparoxysmal forms of sinus node tachycardia, often referred to as inappropriate sinus tachycardia (IST), show similar ECG patterns but can demonstrate a gradual acceleration (warm-up phenomenon), which suggests enhanced automaticity.32 IST is characterized by an elevated heart rate (HR) at rest and an exaggerated HR response to physical activity or emotional stress. These have been reproduced with isoproterenol.
Accelerated junctional rhythms manifesting as supraventricular tachyarrhythmias have been recorded with particular frequency in the era of extensive digitalis use without blood concentration determinations.42 They have been noted in postoperative patients, after myocardial infarction (MI) and during electrolyte abnormalities.42,43 These arrhythmias are believed to be caused by triggered automaticity resulting from delayed afterdepolarizations in most instances. Pacing can enhance the afterdepolarizations, and they can be increased in amplitude by an increase in extracellular calcium concentrations, which can be seen in digitalis toxicity. Ischemia in experimental models can also result in accelerated, triggered automaticity in the coronary sinus region. Hypokalemia can predispose to afterdepolarizations. Acceleration is often seen in triggered rhythms at initiation and can clinically manifest as nonparoxysmal junctional tachycardias on the ECG. The triggered arrhythmias can often be induced by overdrive pacing and do not necessarily resume after pacing ceases, unlike other automatic rhythms. Triggered rhythms have also been induced in human diseased atrial tissues resected at surgery and in coronary sinus cells during experimental studies.
Clinical Presentation
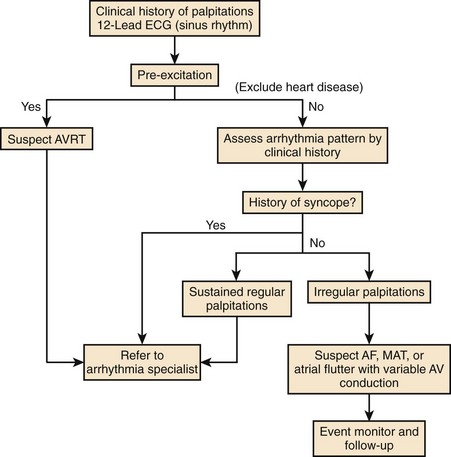
PSVT presents most commonly as sudden onset of palpitations with rates typically ranging from 100 to 260 beats/min and may be associated with chest discomfort, dyspnea, near syncope, and syncope.1,44 It is of variable duration and may last from seconds to days. Termination is usually sudden as well, although in some forms, gradual disappearance may be noted. Chest discomfort in children and adults without overt heart disease may be related to the perception of rapid heart action; after an episode, it may persist in a milder form for a period. In older patients and in the presence of heart disease, this may be related to myocardial ischemia. Dyspnea may be a prominent symptom, often in patients with pre-existing left ventricular dysfunction, when pulmonary congestion may worsen because of poor forward cardiac output. Symptoms suggestive of near-syncope and syncope are seen with extremely rapid PSVT and result from a compromise of cardiac output. In many forms of PSVT, atrial and ventricular activations are not timed sequentially to achieve appropriate ventricular filling.45 Rapid rates further compromise this, and forward cardiac output can be seriously compromised, especially with heart rates greater than 200 beats/min. In some patients, PSVT can be minimally symptomatic or even asymptomatic. In children and infants, incessant PSVT can lead to a tachycardia-induced cardiomyopathy with symptoms of left ventricular failure, failure to thrive, and syncope. Very rapid or hemodynamically unstable episodes of this arrhythmia have been known to precipitate MI, ventricular tachyarrhythmias, and cardiac arrest. SART is a form of paroxysmal SVT characterized by palpitations, and tachycardia rates slower than AVNRT or AVRT, typically ranging from 100 to 150 beats/min.46 Gomes reported a history of recurrent palpitations with a significant proportion having syncope or dizzy spells.46 Most had organic heart disease. IST is a form of PSVT.47 It is an ill-defined clinical syndrome with diverse clinical symptoms that can range from intermittent palpitations to multi-system complaints.47,48 A clinical scheme for evaluation of symptomatic PSVT from the American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) practice guidelines is shown in Figure 41-5.49
Electrocardiography
Onset
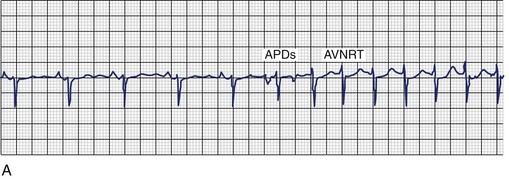
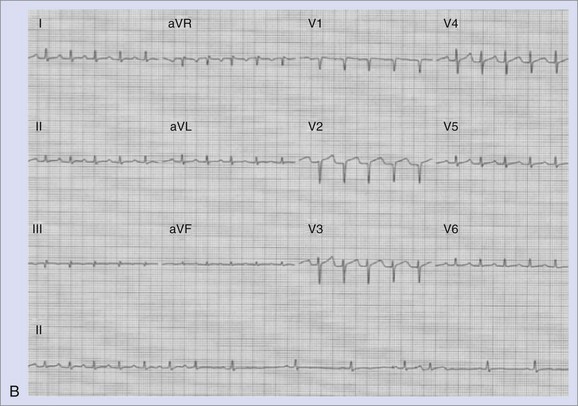
Both SNRT and AVNRT are usually triggered by a premature atrial beat that differs in morphology from sinus rhythm. As shown in Figure 41-6, A, initiation of the tachycardia is characterized by sudden prolongation of the P-R interval because conduction from the premature beat blocks the fast pathway and conducts down the slow pathway. Re-entry occurs if the fast pathway has recovered and is capable of conducting in a retrograde direction. In contrast, a triggered or re-entrant atrial tachycardia (AT) may be initiated by an action potential duration (APD), but these arrhythmias are not heralded by marked P-R interval prolongation with the onset of tachycardia. AVRT mediated by accessory pathways may be triggered by premature atrial or ventricular beats. Electrocardiographic findings in SNRT show an antegrade upright P wave during the tachycardia, morphologically similar to the P wave in sinus rhythm. Figure 41-6, B, shows an electrocardiogram from a patient with paroxysmal sinus tachycardia that was refractory to antiarrhythmics. Coexistence of sinoatrial re-entrant tachycardia with AVNRT has been observed. Triggering with both atrial and ventricular premature beats has been described. Ventricular ectopy can induce AVRT or AT, but it is a far less common mode of induction for these arrhythmias.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree