Introduction and historical perspective
The development and implementation of the first fully implantable cardiac pacemaker in 1958 transformed the outlook for patients with symptomatic bradycardia and Stokes–Adams attacks.1 Since then, technologic advances and innovation over five decades have enabled the development of increasingly sophisticated pacing systems, better able to simulate the normal cardiac activation sequence, and a wide variety of different pacing modes is now available.
Initially, pacemakers were only implanted for atrioventricular (AV) block, but their use was soon extended to the management of symptomatic bradycardia associated with sinus node disease. In recent years, improved understanding of pathophysiologic mechanisms has prompted the assessment of pacemaker therapy in other conditions such as neurocardiogenic syncope, hypertrophic cardiomyopathy and paroxysmal atrial fibrillation. Most recently, the development of cardiac resynchronization therapy using biventricular pacing has emerged as an important treatment for selected patients with heart failure. With the emergence of new indications for pacing and the availability of a vast array of different pacing modes and techniques, an evidence-based approach to the practice of cardiac pacing has become increasingly important.
The fundamental aims of cardiac pacing are to relieve symptoms, to improve the quality of life and, in some instances, to prolong survival. The achievement of these aims is mediated by improvements in hemodynamic function and functional capacity, reduction in cardiovascular morbidity, and prevention of sudden death. Any consideration of the utility and optimal mode of pacing must have regard to all of these factors. It is important to note that hemodynamic differences between alternative pacing modes do not always translate into significant differences in clinical outcomes and comprehensive assessment in randomized clinical trials is therefore essential. It is also important to bear in mind that inappropriate pacing or complications from pacing may result in new or worse symptoms and increased cardiovascular morbidity. This is perhaps best exemplified by the pacemaker syndrome.2
The most recent world survey of cardiac pacing reported that there were over 540 000 new pacemaker implants in the 43 contributing countries in 20053 and the number of new implants is continuing to rise.3–5 There is considerable national and regional variation in the implant rate. In the USA in 2005, there were approximately 752 new implants per million population. In Europe, figures ranged from 101 per million (Russia) to 789 per million (Belgium).3 These variations may partly reflect differences in the age distribution and morbidity of the relevant populations but availability of resources and variations in standards of medical care and attitudes to pacing may also be relevant. There has also been some suggestion, in the past, of inappropriate and excessive pacemaker implantation.6
In an effort to define appropriate pacing practice, a joint task force subcommittee of the American College of Cardiology (ACC) and the American Heart Association (AHA) has published guidelines for permanent pacemaker implantation, most recently in 2008 (Table 41.1).7 Similar guidelines have also been published in Europe by a task force of the European Society of Cardiology (ESC), in collaboration with the European Heart Rhythm Association.8
Table 41.1 ACC/AHA/HRS guidelines: indications for permanent cardiac pacing7
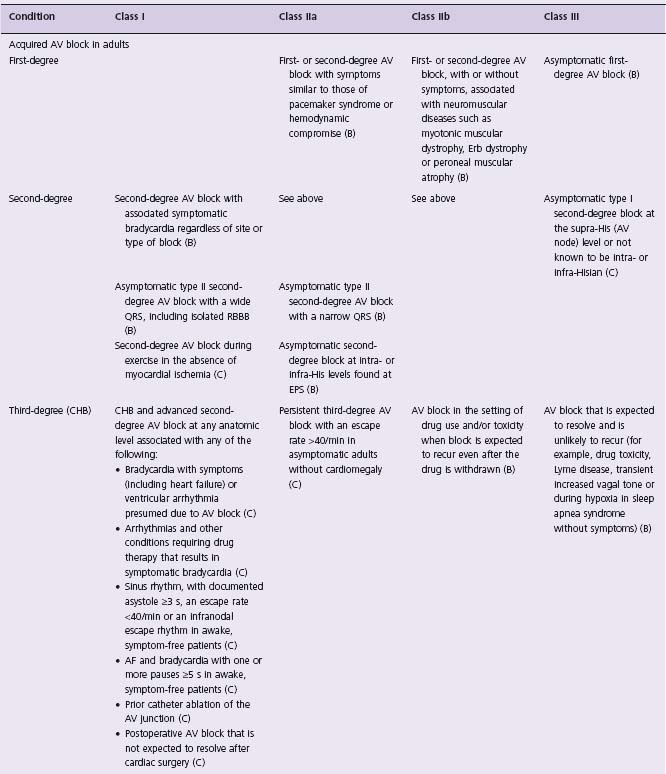
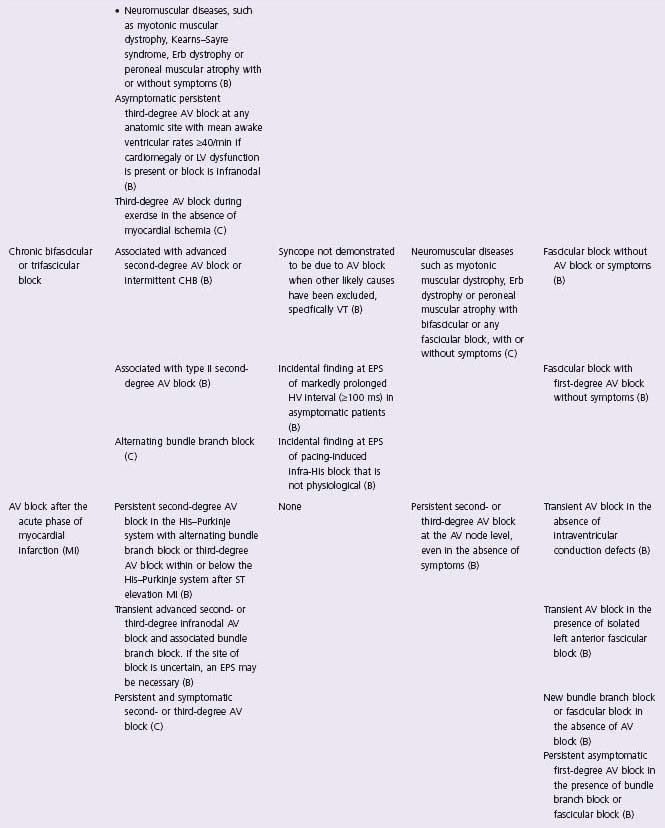
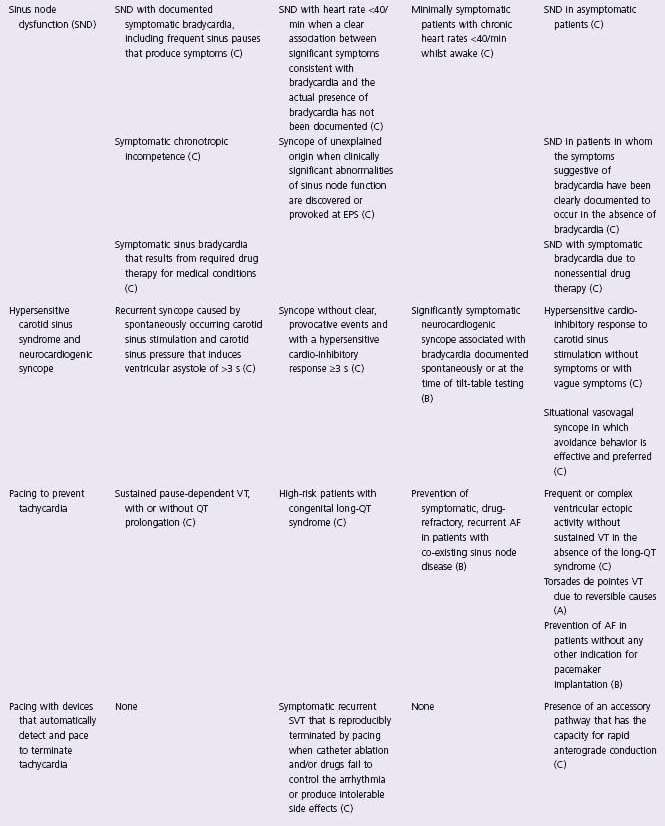

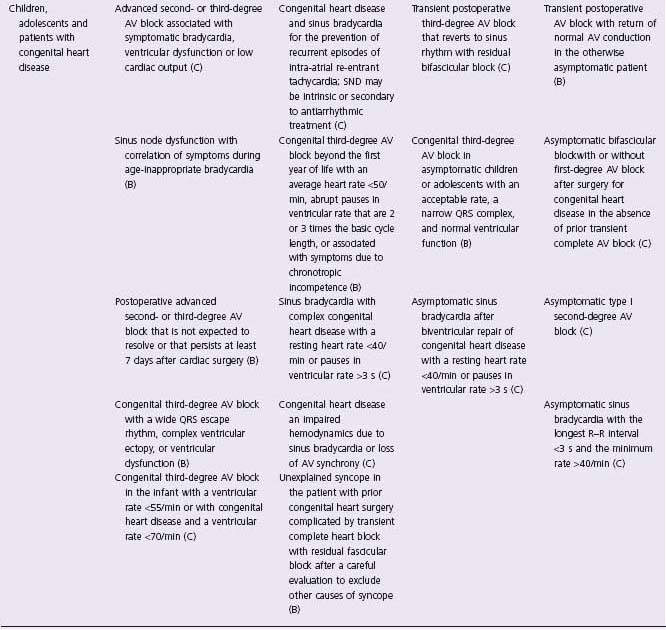
CHB, complete heart block; CHF, congestive heart failure; EPS, electrophysiology study; VT, ventricular tachycardia; RBBB, right bundle branch block.
The grade of evidence supporting each recommendation is indicated in parentheses:
(A) data derived from multiple randomized clinical trials involving a large number of individuals
(B) data derived from a limited number of trials involving comparatively small numbers of patients or from well-designed data analyses of non-randomized studies or observational data registries
(C) recommendation based on consensus opinion of experts.
Class I – conditions for which there is evidence and/or general agreement that pacing is beneficial, useful and effective.
Class II – conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of pacing.
Class III – conditions for which there is evidence and/or general agreement that pacing is not useful/effective and in some cases may be harmful.
Class II is subdivided into Class IIa–weight of evidence/opinion is in favor of usefulness/efficacy and Class IIb–usefulness/efficacy is less well established by evidence/opinion.
Based on Epstein et al.7
Conventional indications for pacing
The principal indication for cardiac pacing is to relieve or prevent symptoms associated with bradycardia. In high-grade AV block, however, there is evidence that survival may also be improved by pacing, even in the absence of symptoms, and pacing should be considered on prognostic grounds alone.
Where significant symptoms are clearly associated with documented bradycardia, the requirement for pacing will rarely be in doubt. In other contexts, the cause of symptoms may be unclear and it is important to note that the most common symptoms are non-specific and prevalent in the elderly population, even in the absence of bradycardia.
The most common causes of bradycardia requiring pacing are impaired impulse formation, as in sinus node disease, or a disturbance of cardiac conduction, as in AV block. These conditions account for the vast majority of primary pacemaker implants.3 The remainder includes patients paced for a variety of conditions, including carotid sinus syndrome, cardio-inhibitory forms of neurocardiogenic syncope and others.
Atrioventricular block
First-degree atrioventricular block
Isolated prolongation of the PR interval may be a normal variant in healthy young subjects.9 In older subjects, it is more often associated with underlying pathology, such as conducting system fibrosis or coronary artery disease, but it does not usually give rise to symptoms and pacing is not generally indicated.
Occasionally, symptoms may arise if the PR interval is markedly prolonged. Atrial systole may then closely follow delayed ventricular systole from the previous cycle, resulting in a comparable hemodynamic disturbance to that seen in the pacemaker syndrome10,11 and a favorable response to dual-chamber pacing has been reported.12 Symptomatic first-degree AV block with a demonstrable improvement during temporary dual-chamber pacing may reasonably be considered at least a Class IIa13 and perhaps even a Class I14 indication for pacing (Level B).
Second-degree atrioventricular block
When second-degree AV block of any type is associated with clearly attributable symptoms, pacing is indicated. In the absence of symptoms, the situation is more complex. Prognosis is thought to relate to the site of block, proximal block at the level of the AV node being more benign than distal block in the His–Purkinje system.15 The ECG classi-fication into Mobitz type I (Wenckebach), Mobitz type II or advanced (2:1, 3:1 or 4:1) second-degree AV block is purely descriptive and the site of block cannot always be inferred although electrophysiologic studies have shown that type I block is most commonly proximal whereas type II block is almost always distal.16 In the past, type I second-degree AV block has often been regarded as benign but evidence from the Devon Heart Block and Bradycardia Survey (a non-randomized, observational study) suggests that in unpaced patients aged 45 years or over, even amongst those without attributable symptoms, there is an increased incidence of adverse outcomes (symptomatic bradycardia, higher degree AV block or impaired survival) compared to the general population and that pacing is associated with improved survival.17,18 Guidelines differ in their conclusions but published opinion suggests that pacing should be considered in asymptomatic type I second-degree AV block, particularly in older patients with structural heart disease19,20 ( Class IIa, Level C). In young subjects, however, asymptomatic type I second-degree AV block occurring during sleep or associated with athletic training is more likely to reflect high resting vagal tone and pacing is unnecessary.21,22 In type II second-degree AV block, progression to complete AV block is common, particularly when the QRS complex is wide,15 and pacing is indicated even in the absence of symptoms (Class I, Level C).
Complete atrioventricular block
In symptomatic complete AV block, pacing usually, although not invariably, improves the symptoms and should always be considered. Irrespective of symptoms, however, untreated acquired complete heart block is associated with significantly impaired survival. Overall mortality may exceed 50% at 1 year, the outlook being worse in older patients ( > 80 years) and those with associated non-rheumatic structural heart disease.23 Male sex and a history of syncope have also been associated with a worse outlook in some studies24 but there is conflicting evidence regarding syncope.25 Transient AV block carries a more favorable prognosis, with a 1-year mortality of 36%, compared with 70% in patients with permanent AV block,23 but a significant proportion of patients (38–39% over median follow-up of 36–54 months) progress to permanent AV block and become pacemaker dependent when paced.26
Observational studies of outcome in paced patients during the early days of cardiac pacing suggested that pacing in complete AV block could improve survival to approach that of a similar age-and sex-matched group.24 Mortality was higher in those with a history of myocardial infarction but not influenced by pre-pacing QRS duration or morphology, ventricular rate (dichotomized about 40/ min) or whether AV block was intermittent or constant.27 In a more recent study of patients aged ≥ 65 years, paced for symptomatic, high-grade AV block, overall survival was less than expected for an age-and sex-matched cohort.28 However, in patients aged < 80 years without structural heart disease, survival was normal. Congestive heart failure, chronic obstructive pulmonary disease, age, syncope, insulin-dependent diabetes and male gender emerged as independent predictors of increased mortality. There have been no prospective randomized trials to assess the impact of pacing in high-grade AV block but the prevalence of symptoms, the high mortality without pacing, the strength of the data from observational studies, and the absence of any alternative therapy suggest that such a trial is neither ethical nor necessary. The vast majority of patients with complete AV block should be paced, whether or not they have symptoms (Class I, Level C)
Congenital complete atrioventricular block
In patients surviving to adulthood, the prognosis of congenital complete AV block has previously been regarded as benign, based largely on retrospective studies of a small series of patients.29 More recent data concerning long-term follow-up (7–30 years) of 102 patients with isolated congenital complete AV block, who survived without symptoms to the age of 15 years, suggests a less favorable outlook.30 Stokes–Adams attacks occurred in 27 patients, of whom eight died (six during the first attack) and six others required cardiac resuscitation. All survivors received pacemakers. A further eight patients had repeated fainting spells requiring pacing and 27 others were paced for other reasons (fatigue, effort dyspnea, dizziness, ectopics during exercise, mitral regurgitation or slow ventricular rates). Of 40 patients followed for 30 years, only four remained asymptomatic without pacing. The only significant predictor of risk was QTc prolongation, which was seen in seven patients, all of whom had Stokes–Adams attacks and three of whom died. In contrast to previous studies, low ventricular rates, widened QRS complexes, poor chronotropic response to exercise and ectopics were not predictive of future Stokes–Adams attacks or death. These data support the authors ’ recommendation of prophylactic pacing in adolescents and adults with congenital complete AV block, even without symptoms, notwithstanding the fact that a number of questions remain unanswered (Class IIa, Level C).31
Fascicular block
In asymptomatic subjects with unifascicular block (right bundle branch block, left anterior hemiblock or left posterior hemiblock), the risk of progression to high-grade AV block is remote32 and pacing is not indicated (Class III, Level C). In asymptomatic bifascicular block (left bundle branch block or right bundle branch block with left anterior or posterior hemiblock), the risk of progression to high-grade AV block is in the region of 2% per annum. Prognosis is principally determined by the presence or absence of underlying structural heart disease and prophylactic pacing is not routinely indicated (Class III, Level B).33 Progression to high-grade AV block is more commonly seen in patients with a history of syncope but should not be presumed to be the cause without further assessment. If high-grade AV block is documented, pacing is mandatory (Class I, Level B). When the cause of syncope remains unclear, an electrophysiology study may help identify patients likely to benefit from pacemaker implantation. A prolonged HV interval >100ms and His–Purkinje block during atrial pacing have high specificity for prediction of subsequent progression to high-grade AV block.34,35 Less marked HV prolongation ( > 70 ms) is more common but its significance is uncertain.36 Sensitivity for disclosure of latent high-grade AV block may be markedly enhanced by the use of intravenous disopyramide during the study but this is not advised in patients with impaired left ventricular (LV) function.37 The electrophysiology study may also be of value to identify inducible ventricular tachycardia,38 which argues against the empiric use of permanent pacing in this context. However, in patients with bifascicular block and a history of syncope for which no other cause is apparent despite thorough evaluation, including an electrophys-iology study, empiric pacing may be the most expeditious course. This strategy is principally justified for relief of symptoms as pacing does not appear to influence mortality or the incidence of sudden death in this context (Class IIa, Level B).33
Atrioventricular and bundle branch block after myocardial infarction
Transient conduction disturbance is a relatively common complication of acute myocardial infarction. Long-term prognosis is principally determined by the extent of myocardial injury. When AV block complicates inferior myo-cardial infarction, it typically resolves within a few days and rarely persists beyond 2 or 3 weeks. In anterior infarction, however, AV block may reflect extensive septal necrosis and the prognosis is poor despite pacing.39 Patients with high-grade AV block persisting for more than 3 weeks after myocardial infarction should be considered for permanent pacing (Class I, Level C).
The occurrence of an intraventricular conduction disturbance (apart from isolated left anterior hemiblock) in patients with acute myocardial infarction identifies a group with poor short-term and long-term prognosis and an increased risk of sudden death.40 The poor prognosis in this group, however, is mainly attributable to a high incidence of malignant ventricular arrhythmia, pump failure, and electromechanical dissociation, rather than progressive conduction disturbance. A prospective study of 50 patients randomized to pacing or control groups and followed for 5 years showed no significant difference in survival.41 However, evidence from a retrospective multicenter study of patients with bundle branch block complicating myocardial infarction indicates that transient high-degree AV block during the acute phase is associated with a high incidence of recurrent AV block and sudden death that may be reduced by pacemaker implantation.42,43 The risk appears to be particularly high in patients with block involving the right bundle and at least one fascicle of the left bundle (Class I, Level B).43,44
Sinoatrial disease
Sinoatrial disease encompasses a wide spectrum of arrhythmia including sinus bradycardia, sinus arrest, sino-atrial block, sick sinus syndrome, the tachycardia–bradycardia syndrome and chronotropic incompetence (failure to reach at least 80% of the maximum predicted heart rate at peak exertion).45 The prognosis in sinoatrial disease is generally good unless myocardial ischemia, heart failure or systemic embolism is present.46 Permanent pacing is indicated for the relief of symptoms that are due to bradycardia.
The efficacy of pacing in sick sinus syndrome has been assessed in a randomized trial.47 One hundred and seven patients with symptomatic sick sinus syndrome were randomized to receive no treatment, oral theophylline or permanent dual chamber adaptive rate (DDDR) pacing. Patients were excluded in very severe cases, defined as symptomatic resting sinus rate < 30/min, sinus pauses >3s or heart failure refractory to treatment with angiotensin-converting enzyme (ACE) inhibitors and diuretics. During a mean follow-up period of 19 months, both pacing and theophylline were associated with a lower incidence of heart failure compared with no treatment (3%, 3% and 17% respectively) but only pacing was associated with a significantly lower incidence of syncope (6%, 17% and 23% respectively). Pacing remains the treatment of choice for patients with symptomatic sick sinus syndrome (Class I, Level B/C). In the absence of long-term follow-up data to confirm efficacy and safety, theophylline or other phar-macologic means of chronotropic support cannot be recommended.
Pacing does not appear to improve survival in sinoatrial disease48 and it is not generally indicated in asymptomatic patients (Class III, Level C). Such patients, however, should be followed closely to assess progression. Athletically trained subjects may have sinus rates as low as 30/ min during sleep with pauses of almost 3 s.49 These findings usually reflect high vagal tone and do not require pacing in the absence of symptoms. If lower rates or longer pauses are observed during sleep or if similar findings occur during the day, particularly if there is evidence of progression with time, prophylactic pacing may be justified on empiric grounds although there are no supportive data (Class IIb, Level C).
Mode selection in AV block and sinus node disease
In AV block, the essential requirement is that the ventricle be paced. When sinus rhythm and chronotropic competence are preserved, dual-chamber pacing with atrial tracking will ensure the maintenance of AV synchrony and physiologic rate adaptation. When sinus rhythm is absent or when chronotropic incompetence is present, an extrinsic sensor may be used to provide rate adaptation with either ventricular or dual-chamber pacing, as appropriate. In isolated sinoatrial disease, rate support can be achieved by single-chamber pacing in the atrium or ventricle, or by dual-chamber pacing.
Both dual-chamber pacing and adaptive rate single-chamber pacing have been shown to offer benefits in terms of improved hemodynamics, increased treadmill exercise tolerance and reduced symptoms when compared with single rate ventricular pacing in small randomized crossover studies.50–58 The mean patient age in most of these studies was younger than the typical paced population although similar benefits have also been reported in patients aged 75 years or over.59 Nonetheless, the long-term clinical benefit of physiologic pacing in the elderly has been questioned.60 Small-scale quality of life studies have yielded conflicting results although physiologic pacing, with preservation of AV synchrony and rate adaptation, does appear to offer advantages in terms of symptoms and there is considerable evidence of patient preference for physiologic modes.61 Single-chamber ventricular pacing is associated with an increased risk of pacemaker syndrome, which has been estimated to occur in between 7% and 20% of patients.2 It has, however, been suggested that a subclinical form may be present in many apparently asymptomatic patients.62 Data from retrospective studies suggest that ventricular pacing is associated with an increased risk of atrial fibrillation (AF), heart failure, and thromboembolism.46,63 and with increased mortality in some patient groups.64–66
The Danish trial
reported by Anderson and colleagues, was the first prospective randomized trial of pacemaker mode selection.67 In this study, 225 patients with sick sinus syndrome were randomized to either single-chamber atrial (AAI) or single-chamber ventricular (VVI) pacing and followed for a mean of 3.3 years. Neither the incidence of AF or stroke nor survival differed significantly between the two groups, although the incidence of a combined end-point of stroke plus peripheral embolism was significantly lower in the atrial paced group. Extended follow-up of the same group of patients was reported after a mean of 5.5 years.68 The previously identified benefits of atrial pacing were enhanced, with a significantly lower incidence of AF, thromboembolism and heart failure in the atrial paced group. All-cause mortality and mortality due to cardiovascular causes were also significantly lower in the atrial paced group. Only four of 110 patients in the atrial paced group developed second-or third-degree AV block, requiring pacemaker upgrade (0.6% per annum).69
The Pacemaker Selection in the Elderly (PASE) study
randomized 407 patients, aged 65 or older (mean age 76 years), to ventricular or dual-chamber pacing.70 All patients received DDDR pacing systems and mode randomization was achieved by programming of the pacemaker. The group included 175 patients with sinus node disease, 201 patients with AV block and 31 patients with other diagnoses. The study was powered to assess differences in health-related quality of life. As would be expected, there was marked improvement in quality of life (SF-36) after pacemaker implantation but there were no significant differences between groups in relation to pacing mode. It is noteworthy that 26% of the patients randomized to ventricular pacing crossed over to dual-chamber pacing because of symptoms attributed to pacemaker syndrome. Whilst potentially significant in itself, the high crossover rate confounds interpretation of the data, particularly in respect of clinical outcomes. In a multivariate analysis, a decrease in systolic blood pressure to <110mmHg during ventricular pacing at the time of pacemaker implantation ( P = 0.001), use of beta-blockers at the time of randomization ( P = 0.01) and non-ischemic cardiomyopathy (P = 0.04) were the only variables that predicted crossover.71
The Canadian Trial of Physiologic Pacing (CTOPP),
the largest reported to date, included 2568 patients aged 18 years or older (mean age 73 years), with either AV block (60%) or sinus node disease (40%), who were randomized to receive either a ventricular (VVIR) or a physiologic pacemaker.72–74 In the physiologic arm, investigators selected either an atrial (AAIR) or a dual-chamber (DDDR) system. Adaptive rate pacing was used in both groups if chrono-tropic incompetence was evident and in patients with complete AV block randomized to receive ventricular pacing. Over a mean follow-up of 3 years there was no significant difference in the primary outcome of cardiovascular death or stroke (VVIR 5.5% per annum vs physiologic 4.9% per annum; relative risk reduction 9.4%; 95% confidence interval (CI)-10.5 to 25.7; P = 0.33). Neither was there any significant difference in all-cause mortality or in hospital admission for heart failure. There was, however, a significant, albeit modest reduction in AF (defined as an episode lasting more than 15 minutes) associated with physiologic pacing (VVIR 6.6% per annum vs physiologic 5.3% per annum; relative risk reduction 18.0%; 95% CI-0.3 to 32.6; P = 0.05), which became evident after about 2 years. Peri-operative complications were more common with physiologic pacing (VVIR 3.8% vs physiologic 9.0%; P < 0.001) mainly in relation to the pacing lead(s). There was a trend suggesting that younger patients ( < 74 years) might have a reduced risk of stroke or cardiovascular death with physiologic pacing.
Subgroup analysis of the CTOPP data suggests that the benefits of physiologic pacing may be influenced by pacemaker dependency75 This was assessed in 87% of the enrolled patients by recording the unpaced heart rate at the first follow-up visit (2-8 months post implant). In patients with unpaced heart rates <60/min, the incidence of cardiovascular death or stroke was lower with physiologic pacing (VVIR 6.4% per annum vs physiologic 4.1% per annum; relative risk reduction 35.5%; 95% CI 12 to 53). By contrast, the treatment effect of physiologic pacing was slightly negative in patients with unpaced heart rates > 60/min (VVIR 4.1% per annum vs physiologic 4.3% per annum; relative risk reduction —1.9%; 95% CI-50 to 31). The difference in treatment effect between the two groups was of only borderline significance ( P = 0.058).
Quality of life improved with pacing but there were no significant differences according to pacing mode irrespective of pacemaker dependency76 A limited economic analysis in a subset of 472 patients showed that physiologic pacing was not cost-effective according to generally accepted standards unless used selectively in patients likely to be pacemaker dependent.77
The CTOPP investigators subsequently reported the results of extended follow-up after a mean of 6.4 years. The previously observed difference in the rate of AF persisted, with a relative risk reduction in the physiologic group of 20.1% (95% CI 5.4 to 32.5; P = 0.009) but there was no significant difference in the rate of cardiovascular death or stroke.78
The Mode Selection Trial (MOST)
assessed the benefits of adaptive rate, dual-chamber pacing compared with adaptive rate, single-chamber, ventricular pacing in 2110 patients aged >21 years, with sinus node dysfunction.79 A DDDR pacing system was implanted in all patients and the pacing mode was randomized to VVIR or DDDR. After a median follow-up of 33.1 months, there was no difference in the incidence of the primary endpoint, death or non-fatal stroke, between the groups (VVIR 23.0% vs DDDR 21.5%; P = 0.48).80 However, dual-chamber pacing was associated with a lower incidence of AF (hazard ratio (HR) 0.79; 95% CI 0.66 to 0.94; P = 0.008). Heart failure scores were also significantly improved but this did not result in a lower incidence of hospitalization for heart failure in the primary unadjusted analysis (VVIR 12.3%; DDDR 10.3%; HR 0.82; 95% CI 0.63 to 1.06; P = 0.13). Quality of life was substantially improved by pacing with a small but significant advantage for the dual-chamber mode in three of the eight subscales of the SF-36.81 Crossover from VVIR to DDDR mode occurred in 374 patients but 61 patients subsequently switched back, resulting in a crossover rate at final follow-up of 31.4%. Almost half of the crossovers were attributed to pacemaker syndrome. The high crossover rate in PASE and MOST, where a mode change only required reprogramming of the device, contrasts with the low rates in CTOPP (2.7% at 3 years) and UKPACE (3.1% at 3 years), in which replacement of the pacing system would have been required. Although the high crossover rate in MOST complicates interpretation of the data, an analysis based on treatment received showed that it had no impact on the primary endpoint or other key clinical outcomes.82 An economic analysis suggested that dual-chamber pacing in this patient group is cost-effective at conventionally accepted levels both within the 4-year time horizon of the trial and projected over the lifetime of the patients.83
The United Kingdom Pacing and Cardiovascular Events (UKPACE)
trial enrolled patients aged ≥ 70 years with high-grade AV block. Prior to the trial, national registry data showed evidence of ageism in clinical practice, with elderly patients being much less likely to receive dual-chamber pacemakers than those who were younger.84 In the trial, 2021 patients were randomly assigned to receive either single-chamber or dual-chamber pacemakers and followed for a minimum of 3 years. In the single-chamber arm, assignment to VVI or VVIR was also randomized. After median follow-up of 4.6 years, there was no significant difference in the primary outcome of all-cause mortality (VVIR 7.2% per annum vs DDDR 7.4% per annum; HR 0.96; 95% CI 0.83 to 1.11; P = 0.33). Median follow-up for other events was 3 years and showed no significant difference in the rates of AF, heart failure or a composite of stroke, transient ischemic attack (TIA) or other thromboembolism.85 When VVI and VVIR pacing were compared separately with dual-chamber pacing, there was a significantly higher rate of stroke, TIA or other thromboembolism with VVI pacing than with DDD (2.5% per annum vs 1.7%; P = 0.04) but there were no other significant differences. Complications related to the implantation procedure were more common in the dual-chamber group than in the single-chamber group (7.8% vs 3.5%; P < 0.001), as were complications before hospital discharge (10.4% vs 6.1%; P < 0.001). Preliminary analysis of quality of life showed improvement with pacing but no significant difference according to randomized pacing mode. Suspected pacemaker syndrome occurred in only 2.7% of those receiving single-chamber pacing86 and crossover from single-to dual-chamber pacing in 3.1%.85
The Danish Multicenter Randomized Study on Atrial Inhibited versus Dual-Chamber Pacing in Sick Sinus Syndrome (DANPACE)
aims to examine the relative merits of single-lead atrial pacing (AAIR) and dual-chamber pacing (DDDR) in patients aged ≥ 18 years with sick sinus syndrome (including tachycardia-bradycardia syndrome) and normal AV conduction.87 The primary outcome measure is all-cause mortality. Secondary outcomes include cardiovascular mortality, AF, thromboembolism, quality of life and cost–benefit. Recruitment of 1415 patients (reduced from an original target of 1900) was completed in June 2008 and follow-up will be concluded in 2009.
A prior pilot study randomized 177 patients with sick sinus syndrome and normal AV conduction to single-chamber atrial pacing (AAIR), or dual-chamber pacing (DDDR) with either a short AV delay or a fixed long AV delay. During a mean follow-up of 2.9 years, echocardiog-raphy showed no significant changes in left atrial or ventricular diameter or in LV fractional shortening in the atrial pacing group. However, left atrial diameter increased in both of the dual-chamber groups and LV fractional shortening decreased in the dual-chamber group with short AV delay.88 The incidence of AF was significantly lower in the atrial pacing group (7.4%) than in the dual-chamber groups with short (12.3%) or long (17.5%) AV delay, the benefit being most evident in patients with brady-tachy syndrome.89
The benefits of physiologic pacing have been addressed in four other trials for which only limited data are available. An Italian study reported on 210 patients with high-grade AV block (100 patients) or sick sinus syndrome (110 patients) with no prior AF, randomly assigned to ventricular (VVI or VVIR) or atrial-based (AAI, DDD, DDDR or VDD) pacing.90 The incidence of chronic AF was lower with atrial-based than with ventricular pacing, particularly in those with sick sinus syndrome. The Pacemaker Atrial Tachycardia (PAC-A-TACH) trial assessed the effect of pacing mode on atrial tachyarrhythmia recurrence in 198 patients with the tachycardia-bradycardia syndrome.91 All patients received dual-chamber, adaptive rate pacemakers programmed to either VVIR or DDDR pacing. After a median of 23.7 months follow-up, 44% of patients crossed over from VVIR to DDD (due to pacemaker syndrome in 28% and atrial tachyarrhythmia in 13%) and 9% crossed over from DDDR to VVIR (due to recurrent atrial tachyar-rhythmia in 7% and atrial lead problems in 2%). Intention-to-treat analysis showed no significant difference in atrial tachyarrhythmia recurrence rates at 1 year (VVIR 43%; DDDR 48%; P = 0.09). Mortality, a secondary outcome, was significantly higher in the VVIR group and the trial was stopped after follow-up of approximately 2 years in all patients. Cumulative mortality was 21% in the VVIR group and 5% in the DDDR group ( P < 0.001). Pacing mode (risk ratio 4.3; 95% CI 1.6 to 11.4; P = 0.004) and prior history of myocardial infarction (risk ratio 3.1; 95% CI 1.4 to 6.7; P = 0.006) were identified as independent predictors of mortality.92 The Systematic Trial of Pacing to Prevent Atrial Fibrillation (STOP-AF) randomly assigned 227 patients with sick sinus syndrome, all implanted with a DDDR pacing system, to either atrial-based (AAI or DDD) or ventricular pacing.93,94 The results have not yet been presented or published. Finally, the ongoing Pacing the Octagenarian Plus (POPP) trial is comparing the effect of single-chamber (VVIR) and dual-chamber (DDDR) pacing on hospitaliza-tion for cardiovascular causes, functional capacity and quality of life in the very elderly.95
A prospectively planned meta-analysis of patient-level data from five of the trials (the Danish Trial, PASE, CTOPP, MOST and UKPACE) demonstrated a reduction in AF with atrial-based pacing (HR 0.80; 95% CI 0.72 to 0.89; P = 0.00003) and in stroke (HR 0.81; 95% CI 0.67 to 0.99; P = 0.035).96 There was no significant reduction in mortality or heart failure and no convincing evidence that any patient subgroup derived special benefit from atrial-based pacing. The key findings are shown in Figure 41.1.
Figure 41.1 Effect of pacing mode, expressed as the HR and 95% CI. An HR < 1.0 is shown to the left of the center line and favors atrial-based pacing. CIs that cross 1.0 signify a statistically non-significant effect. (a) All-cause mortality. (b) Incidence of atrial fibrillation. (c) Incidence of stroke (Reproduced with permission from Healey et al.96)
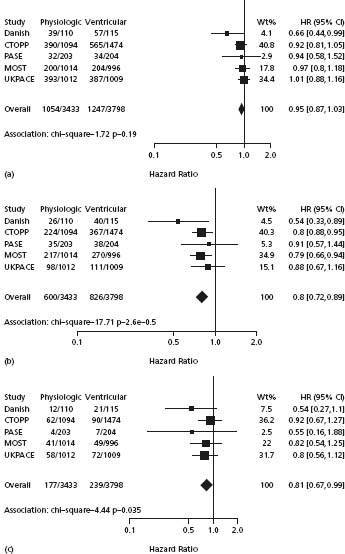
Two systematic reviews have comprehensively assessed the various parallel group and crossover randomized controlled trials comparing single-chamber ventricular pacing and dual-chamber pacing.97,98 The aggregated data indicate an overall advantage for dual-chamber pacing, related to the lower incidence of AF and the reduction in pacemaker syndrome. These benefits must be weighed against the increased implant complications and cost. The overall estimate of cost-effectiveness of dual-chamber pacing varied between around UK £ 9000 and around UK £ 30 000 per quality-adjusted life-year, according to the assumptions made about the incidence and severity of pacemaker syndrome.98
A new paradigm for physiologic pacing
The results of the various prospective randomized trials suggest that the clinical benefits of physiologic or dual-chamber pacing may previously have been overestimated. Pacemaker recipients are often elderly with multiple co-morbidities and a high mortality from non-cardiovascular causes. Against this background, it may be unrealistic to expect a marked effect of pacing mode on quality of life, clinical events and mortality, all of which may be more influenced by other factors. A further explanation for the limited clinical benefits of physiologic pacing might be that the conventional placement of the ventricular lead at the right ventricular apex causes interventricular and intraven-tricular dyssynchrony that could offset the hemodynamic advantage of preserved AV synchrony. It has long been recognized that eccentric ventricular stimulation results in impaired LV performance.99 Pacing at the right ventricular apex produces an activation sequence similar to that of left bundle branch block, the acquired form of which is associated with an adverse prognosis particularly in patients with heart failure.32,100,101 Whilst this may partly reflect advanced underlying disease, there is some evidence that the conduction disturbance is an independent marker of adverse outcomes.101 Paradoxically, the problem may be accentuated by dual-chamber pacing. In an analysis of patients in the MOST study with normal baseline QRS duration, the cumulative percentage of ventricular beats that were paced was greater with dual-chamber than with single-chamber pacing and a higher cumulative percentage of ventricular pacing was associated with an increased risk of heart failure hospitalization and AF with either pacing mode.102 Further data suggesting a deleterious effect of right ventricular apical pacing have emerged from studies of pacing strategies in implantable cardioverter defibrilla-tor (ICD) recipients. In the Dual-chamber and VVI Implant-able Defibrillator (DAVID) trial, 506 patients with an ICD capable of dual-chamber adaptive rate pacing were randomly assigned to back-up ventricular pacing at 40/min or dual-chamber adaptive rate pacing at 70/min. All patients had ejection fraction ≤ 40% but no indication for cardiac pacing. Over 1 year, the incidence of the composite endpoint of death or hospitalization for heart failure was significantly increased in the dual-chamber paced group (HR 1.61; 95% CI 1.06 to 2.44 ).103 Similarly, in a subgroup analysis of the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II, which tested the benefit of a prophylactic ICD (single-or dual-chamber with back-up pacing) in patients with prior myocardial infarction and LV ejection fraction ≤ 30%, patients who were predominantly paced had an increased incidence of new or worsening heart failure.104
Recognition of the potential importance of dyssynchrony from right ventricular pacing has prompted a re-evaluation of what constitutes “ physiologic ” pacing and a new paradigm is emerging.105,106 The key considerations are that, in addition to preserving AV synchrony, the selected therapy should minimize unnecessary ventricular pacing and minimize intraventricular and interventricular dyssynchrony when ventricular pacing is required.
Minimizing ventricular pacing
In patients with sinus node disease and normal AV conduction, single-chamber atrial pacing permits ventricular activation via the intrinsic conduction system, with complete avoidance of ventricular pacing. Although the subsequent development of AV conduction disturbance is infrequent (circa 1.7% per annum),107 many physicians prefer to implant a dual-chamber system to insure against the risk. It is, however, noteworthy that the Danish study was the only randomized trial to use single-lead atrial pacing as the sole physiologic comparator and showed the greatest clinical benefits from physiologic pacing, including a reduction in mortality. The DANPACE trial will provide further information about the relative merits of the two approaches in due course.
If a ventricular lead is placed prophylactically or due to the presence of intermittent AV conduction disturbance, there are several programming options to minimize ventricular pacing although none will truly emulate normal physiology. Fixed, long AV delays are only partially effective in reducing ventricular pacing and may compromise upper-rate behavior and predispose to pacemaker-mediated tachycardia.108 Other options include the use of the DDIR mode, which provides back-up pacing in the ventricle without tracking the atrial rhythm, and algorithms that permit automatic extension of the AV interval (AV search hysteresis) to favor intrinsic ventricular activation.109,110 A number of manufacturers have recently introduced dual-chamber devices with new algorithms that combine functional AAIR pacing with ventricular monitoring and back-up DDDR pacing, when required. These algorithms have been shown to be effective in reducing the cumulative percentage of ventricular pacing.111,112 The recently reported Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) trial showed a lower incidence of AF with the use of AV search hysteresis and minimal ventricular pacing algorithms than with conventional dual-chamber pacing in patients with sinus node disease and normal AV conduction after a mean follow-up of 1.7 years (HR 0.6; 95% CI 0.41 to 0.88; P = 0.009).113 The programming in the conventional dual-chamber arm of the study resulted in a particularly high frequency of ventricular pacing (median 99%), which may have amplified the apparent benefit of the new algorithms but the study nonetheless added to the evidence suggesting benefit from the avoidance of unnecessary ventricular pacing. Attention has been drawn to a potential disadvantage of the algorithms, which may permit or induce very long PR intervals, resulting in potentially significant AV desynchronization in some patients.114 Further trials are in progress to assess the clinical utility of these new approaches.115–117
An additional consideration when programming devices to minimize ventricular pacing is the use of adaptive rate pacing using extrinsic sensors to determine the pacing rate. The Advanced Elements of Pacing Trial (ADEPT) demonstrated, perhaps surprisingly, that rate modulation using current dual-chamber pacemaker technology does not provide measurable improvements in either quality of life or exercise capacity in patients with a blunted heart rate response to exercise.118 Although there was no difference in the prespecified composite clinical endpoint, rate modulation was associated with a higher frequency of hospital-ization for heart failure. It may be that the advantages of restoring chronotropic competence are attenuated by the deleterious effects of increased ventricular pacing. The findings suggest that sensor-driven adaptive rate pacing should be avoided in the absence of chronotropic incompetence and that cautious programming is advisable when it is used.
Minimizing ventricular dyssynchrony
In patients who have permanent AV block and in those who are likely to require more than occasional ventricular pacing, the intraventricular and interventricular dyssynchrony associated with pacing at the right ventricular apex may be attenuated by the use of alternative or multiple pacing sites in the right ventricle, or biventricular pacing.
Alternative and multisite pacing
Numerous small and relatively short-term studies have shown improved hemodynamic function using pacing sites in the right ventricular outflow tract but results have been inconsistent, probably reflecting differences in patient selection, lead positioning and duration of follow-up.119–121 The septal aspect of the outflow tract appears to be the optimal site122 but the precise location for maximum hemo-dynamic advantage may vary between patients. The attainment of a suitable lead position requires sound knowledge of the anatomy, aided by radiologic and electrocardio-graphic confirmation of optimal lead placement.123 Another approach that has been investigated is the use of direct His bundle or para-Hisian pacing, which would be expected to achieve the most physiologic ventricular activation pattern in patients with intact distal conduction.124–126 The technique has been shown to be feasible and to improve hemodynamic and functional outcomes compared with pacing at the right ventricular apex but accurate lead placement is technically challenging and its role remains uncertain. Bifocal right ventricular pacing, with leads at the apex and in the outflow tract, has also been assessed, predominantly in patients with heart failure and conventional indications for cardiac resynchronization therapy.127–129 Improved hemodynamic and functional outcomes have been demonstrated in the short and medium term and the technique may have a role in this setting for patients in whom transvenous placement of the LV lead is unsuccessful and surgical placement of an epicardial lead is not an option.129
Biventricular pacing
Recognition that the dyssynchrony associated with pacing at the right ventricular apex is analogous to that induced by left bundle brunch block has prompted consideration of biventricular pacing in patients with a bradycardic indication for pacing, NYHA class III or IV heart failure and impaired LV function. Patients requiring pacing for bradycardia were generally excluded from the trials of biventricular pacing in heart failure but extrapolation of their results suggests that it may be an attractive therapeutic option in this setting. It might also be of prophylactic benefit, even in those without heart failure or impaired LV function at the time of implant. The utility of biventricular pacing in patients paced for bradycardia was first demonstrated in observational studies in which patients with class III and IV heart failure, previously implanted with a right ventricular pacing system, were upgraded to a biventricular pacing system. The procedure was shown to be feasible and to result in improved NYHA class, ejection fraction and symptoms or quality of life after 3–6 months ’ follow-up.130,131 In a pilot, randomized, crossover trial in patients with advanced heart failure, requiring replacement of conventional pacing systems, biventricular pacing was associated with improved hemodynamics, NYHA functional class, 6-minute walk distance and quality of life.132
The first randomized trial to assess biventricular pacing as a primary strategy in patients requiring anti-bradycardia pacing was the Post AV Nodal Ablation Evaluation (PAVE) trial, which compared right ventricular apical pacing and biventricular pacing in 184 patients, with or without heart failure or LV impairment, who were undergoing AV node ablation for AF.133 After 6 months follow-up, biventricular pacing was associated with a greater improvement in 6-minute walk distance and ejection fraction was preserved, contrasting with a small fall with right ventricular apical pacing. A subsequent smaller 3-month crossover study in severely symptomatic patients with permanent AF, uncontrolled heart rate or heart failure undergoing AV node ablation showed only modest or no benefit from biventricular or LV pacing compared with right ventricular apical pacing, possibly due to the short follow-up.134 More recently, the Homburg Biventricular Pacing Evaluation (HOBIPACE) compared biventricular pacing with conventional right ventricular pacing in 30 patients with AV block requiring pacing, LV dilation ( ≥60mm) and reduced ejection fraction ( ≤ 45%) in a 3-month crossover design.135 Biventricular pacing was superior with regard to LV function, quality of life and exercise capacity, the benefit being similar for patients in sinus rhythm and in AF.
Biventricular pacing has subsequently been compared with conventional dual-chamber pacing in a randomized parallel group study of 50 patients, unselected with regard to baseline ejection fraction, being paced for high-grade AV block.136 After 12 months follow-up, ejection fraction was preserved, N-terminal pro-BNP fell and NYHA class improved in the biventricular paced group, whereas ejection fraction decreased and N-terminal pro-BNP and NYHA class were unchanged in the dual-chamber paced group.
Further trials are ongoing to assess the benefits of biven-tricular pacing as an alternative to conventional pacing in patients with impaired LV function and mild to moderate heart failure (the Biventricular versus Right Ventricular Pacing in Patients with AV Block (BLOCK-HF) trial),137 without advanced heart failure (the Preventing Ventricular Dysfunction in Pacemaker Patients Without Advanced Heart Failure (PREVENT-HF) study),138 with preserved LV function (the Pacing to Avoid Cardiac Enlargement (PACE) trial),139 and unselected with regard to ejection fraction or heart failure at enrolment (the Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchroniza-tion (BioPace) study).140
Comment and recommendations
The available data suggest that dual-chamber pacing confers a modest advantage over single-chamber ventricular pacing due to the reduced risk of AF, the avoidance of pacemaker syndrome and a possible small benefit in quality of life. Interpretation of the data regarding pacemaker syndrome is complicated by the divergent estimates of mode intolerance between trials in which the mode randomization was achieved by software programming (PASE, PAC-A-TACH and MOST) and those in which it was achieved by hardware selection (the Danish study, CTOPP and UKPACE). Each design has strengths and weaknesses but software randomization trials are more vulnerable to the effect of investigator bias.141 In the software randomization trials, crossover rates ranged from 26% to 44%, whereas in the hardware randomization trials, they did not exceed 5%. The true incidence of mode intolerance is most likely between the two extremes. Notwithstanding these uncertainties, dual-chamber pacing is the recommended mode for the majority of patients with sinus node disease or AV block, except in the presence of permanent AF (Class I/IIa, Level A). In patients with isolated sinus node disease and no evident abnormality of AV conduction on ECG or at implant, single-chamber atrial pacing remains an attractive option and provides the most physiologic pattern of ventricular activation.142 Retrospective analysis of pooled data from 28 studies suggests that the risk of subsequent AV block, is low (0.6% per annum)143 and this is supported by data from the Danish study.69 Many physicians will nevertheless prefer to implant a dual-chamber system with features and programming tailored to provide functional AAIR pacing with minimal unnecessary ventricular activation.144 The choice of pacing mode should take account of individual patient factors and in elderly patients with high-degree AV block, single-chamber ventricular pacing (VVIR) is a reasonable option. Adaptive rate pacing should be provided if there is evidence of chronotropic incompetence but unnecessarily aggressive programming of rate response should be avoided. In patients who are likely to require frequent ventricular pacing, alternative pacing sites, such as the septal aspect of the right ventricular outflow tract, may confer an advantage by minimizing dyssynchrony but further data are required before this approach can be recommended for routine practice. Ongoing trials will clarify the role of biventricular pacing in patients requiring pacing for bradycardia. It is, however, a reasonable option to consider, on an individual basis, in patients with NYHA class III or IV heart failure, a low ejection fraction ( ≤ 35%) and a high anticipated requirement for ventricular pacing (Class IIa, Level B). Consideration should also be given to implanting a combined device in patients with a conventional indication for an ICD.
New indications for pacing Neurocardiogenic syncope
Neurocardiogenic syncope describes the clinical syndromes of syncope resulting from inappropriate autonomic responses, manifested as abnormalities in the control of peripheral vascular resistance and heart rate.145 It is thought to account for the largest proportion of faints in clinical practice. The most common forms are carotid sinus syndrome and vasovagal syncope but other related syndromes include cough, deglutition, and micturition syncope. A systematic approach to evaluate patients with syncope is essential and comprehensive guidelines have recently been published.146,147 Carotid sinus massage148,149 and tilt-table testing150 are useful diagnostic tools in carotid sinus syndrome and vasovagal syncope respectively, enabling abnormal reflex responses to be categorized as cardio-inhibitory (asystole > 3 s, bradycardia or AV block), vasode-pressor (fall in systolic blood pressure >50mmHg) or mixed. This has invited assessment of the utility of cardiac pacing which might be expected to benefit patients with predominantly cardio-inhibitory or mixed responses.
Carotid sinus syndrome
Early reports of pacing in carotid sinus syndrome confirmed its efficacy in some patients but persistent symptoms were seen in those with a significant vasodepressor response or hypotension during ventricular pacing.151 The latter was improved by AV sequential pacing and it was suggested that this was the appropriate mode in patients with mixed responses. Attention has been drawn to the variable natural history of the condition, which may remit spontaneously, and the importance of a control group when evaluating therapy has been emphasized.152 A prospective randomized trial of pacing in patients with severe carotid sinus syndrome randomized 60 patients to pacing (VVI in 18 and DDD in 14 patients) or no therapy (28 patients).153 During a mean follow-up of 36 months, syncope recurred in 16 (57%) of the non-paced group and only three (9%) of the paced group; 19 patients (68%) in the non-paced group were eventually paced because of the severity of symptoms. Pacing is now the treatment of choice in all but the mildest forms of carotid sinus syndrome. Recent evidence suggests that carotid sinus syndrome is underdiag-nosed and that comprehensive assessment of patients presenting with syncope, dizziness or falls may identify a significant number of otherwise unrecognized patients who may benefit from pacing.154
In the Syncope And Falls in the Elderly–Pacing And Carotid sinus Evaluation (SAFE PACE) trial, 24 264 patients with falls or syncope were identified from a total of 71 299 emergency room attendees aged ≥ 50 years during a 29-month period.155 Patients with evident extrinsic or medical explanations for falling and those with cognitive impairment were excluded, leaving a residuum of 3384 non-accidental fallers. Of these, 1624 consented to and underwent carotid sinus massage, yielding 257 patients with cardio-inhibitory or mixed carotid sinus hypersensitivity, of whom 175 (mean age 73 years) were randomized to pacing or no pacing and followed for 1 year, to test the hypothesis that dual-chamber pacing, with a rate drop response algorithm, might reduce the frequency of further falls. Paced patients were significantly less likely to fall (odds ratio 0.42; 95% CI 0.23 to 0.75) and syncope and injurious events were less frequent. A larger, multicenter, randomized controlled trial, SAFE PACE 2,156 was subsequently undertaken to further evaluate these findings in a wider cultural setting and to clarify the relationship between symptoms and arrhythmia by the use of an implantable loop recorder in the non-paced patients. No data from this trial are currently available. However, a small crossover trial, comparing pacing (DDD with rate drop response) and no pacing (ODO mode) for 6 months each in 34 patients with recurrent unexplained falls and carotid sinus hypersensitivity has recently been reported.156a This failed to confirm the benefit from pacing that was observed in SAFE PACE but there was a high attrition rate, with only 25 patients completing both phases, and the study was consequently underpowered and inconclusive.
Comment and recommendations
Recurrent syncope caused by spontaneously occurring carotid sinus stimulation with a cardio-inhibitory response to carotid sinus massage (asystole >3s) is a Class I, Level C indication for pacing. Recurrent syncope without clear provocative events and with a cardio-inhibitory response to carotid sinus massage is a Class IIa, Level C indication for pacing. European guidelines also require that the asystolic response to carotid sinus massage be accompanied by syncope or presyncope. The role of pacing in patients with a history of recurrent falls (without clear evidence of syncope) and cardio-inhibitory carotid sinus hypersensitivity is uncertain and should be considered on an individualized basis (Class IIb, Level B).
Vasovagal syndrome
Pacing has also been evaluated in the so-called “ malignant ” form of vasovagal syndrome, characterized by recurrent syncope with only brief or absent prodromal symptoms. Evidence from several studies using temporary pacing during tilt testing indicates that pacing rarely prevents vasovagal syncope, reflecting the fact that hypotension precedes the onset of bradycardia in most patients. However, dual-chamber pacing does attenuate the evolution of the final and most extreme degrees of hypotension and may thereby prolong the symptomatic presyncopal period in selected patients with a documented cardio-inhibitory component.157 A retrospective review of 37 patients receiving predominantly dual-chamber implanted pacemakers, followed for a mean of 50.2 months, reported symptomatic improvement in 89% with 62% remaining free of syncope and 27% completely asymptomatic. The collective syncopal burden was reduced from 136 to 11 episodes per year.158
The North American Vasovagal Pacemaker Study (VPS)159 randomized patients with a history of frequent syncope ( ≥6 lifetime episodes) and a cardio-inhibitory response on tilt testing to receive either no pacing or DDI pacing with a pacemaker incorporating a specialized rate drop-sensing algorithm designed to detect the characteristic pattern of onset of bradycardia that is seen in vasovagal syndrome. The fall in heart rate is typically more marked than occurs with natural diurnal fluctuation yet less precipitous than that seen at the onset of complete AV block or asystole. On detection of a characteristic rate drop, pacing commences with a high initial intervention rate that gradually decreases.160 It had been intended to enroll 284 patients, but the study was stopped after enrolment of only 54 patients due to substantial benefit in the paced group. During follow-up, syncope recurred in 22% of patients who were paced compared with 70% of those who were not (relative risk reduction 85.4%; 95% CI 59.7 to 94.7; P = 0.000022). There was no significant effect on presyncope (reported by 63% of paced patients and 74% of non-paced patients).
The Vasovagal International Study (VASIS) Group subsequently reported a multicenter European trial of similar design.161 Forty-two patients with at least three syncopal episodes in the preceding 2 years and a cardio-inhibitory response to tilt testing were randomized to DDI pacing with rate hysteresis (n = 19) or no pacing (n = 23). Syncope recurred in only one (5%) of the paced patients but in 14 (61%) of the unpaced patients ( P = 0.0006). The median time to first syncope in the unpaced group was 5 months.
The Syncope Diagnosis and Treatment (SYDIT) study compared the relative efficacy of dual-chamber pacing with a rate drop-sensing algorithm and pharmacologic therapy with atenolol.162 Patients were eligible if they had at least three syncopal episodes in the preceding 2 years and a positive response to tilt testing (syncope with relative bradycardia). The study was terminated after 93 patients had been enrolled, as an interim analysis showed a significant effect in favor of pacing. Syncope recurred in only 4.3% of the paced group (after a median of 390 days) compared with 25.5% of the pharmacologically treated group (after a median of 135 days) (odds ratio 0.133; 95% CI 0.028 to 0.632; P = 0.004).
Less encouraging results have since been reported from two double-blind, placebo-controlled trials. In the second Vasovagal Pacemaker Study (VPS II),163 the inclusion criteria were similar to those of the first VPS but in contrast to that study, VASIS and SYDIT, all patients received a pacemaker and were randomized to DDD pacing with a rate drop response algorithm (n = 48) or no pacing (ODO mode; n = 52). Over 6 months follow-up, syncope recurred in 33% of the paced group, compared with 42% of the non-paced group. The relative risk reduction in time to syncope with DDD pacing was 30% but this was not significant (95% CI-33% to 63%; P = 0.14).
In the Vasovagal Syncope and Pacing (SYNPACE) trial,164 29 patients with severe recurrent vasovagal syncope, reproduced on tilt-testing, all received a pacemaker which was randomly assigned to be programmed on (DDD pacing with rate drop response; n = 16) or off (inactive OOO mode; n = 13). During a median follow-up of 715 days, there was no significant improvement in syncope recurrence (50% of the paced group and 38% of the non-paced group) or in time to first syncope.
The difference in outcomes between the blinded and unblinded trials suggests a significant placebo or “ expectation of benefit ” effect from pacemaker implantation. This is supported by the results of a meta-analysis including data from nine randomized trials of pacing for vasovagal syncope.165 The analysis showed that permanent pacing reduced the risk of recurrent syncope in the unblinded trials (odds ratio 0.09; 95% CI 0.04 to 0.22) but not in the double-blind trials (odds ratio 0.83; 95% CI 0.41 to 1.70). The accumulated data do not support the use of pacing as first-line therapy for vasovagal syncope. However, attention has been drawn to the limitations of the trials, all of which were relatively small, with even the largest (VPS II) being limited in power to rule out a modest benefit from pacing.166,167 There was also variation in the enrolment criteria, including the requirement for a positive (cardio-inhibitory) tilt test. Pacing would only be expected to benefit patients in whom syncopal episodes were wholly or predominantly mediated by bradycardia or asystole. The utility of tilt testing to identify such patients is limited by poor reproducibility and a lack of correlation between the responses to tilt testing and the mechanism of spontaneous syncope.146,168 The use of an implantable loop recorder to document the cardiac rhythm during spontaneous syncope and guide further therapy has shown promise and is being further evaluated in a randomized trial.169,170
Comment and recommendations
Pacing should not be considered as first-line therapy for the majority of patients with vasovagal syncope. The initial focus should be on conservative measures, such as education, reassurance and physical maneuvers, which may be particularly useful when there are prodromal symptoms.146
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


