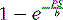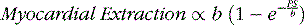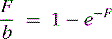Chapter 1 Overview of Tracer Kinetics and Cellular Mechanisms of Uptake
CELLULAR UPTAKE OF MYOCARDIAL PERFUSION AGENTS
Thallium-201
Thallium-201 (201Tl) is a radioactive potassium analog. The initial myocardial uptake of 201Tl is dependent upon myocardial blood flow and its first-pass extraction fraction, which is approximately 85% under resting flow conditions.1,2 At higher flow rates, such as those obtained during pharmacologic vasodilation, the extraction of 201Tl is not linear with respect to flow.3 The plateau in extraction results in an underestimation of the true maximal flow. This phenomenon is true of all diffusible flow tracers and will be discussed in detail in the next section of this chapter.
The intracellular uptake of 201Tl predominantly involves active exchange across the sarcolemmal membrane of the myocytes via the Na+/K+ adenosine triphosphate (ATP) transport system.4 Because this system is energy dependent, thallium transport can only occur in viable myocardium. Once inside the myocyte, 201Tl is not bound intracellularly and can diffuse back out into the circulation. As will be discussed in detail later, these uptake and redistribution kinetic properties form the basis of clinical assessment of myocardial perfusion and viability using 201Tl. Although the introduction of 201Tl in the mid-1970s represented a major advance in nuclear cardiology, its physical properties are not ideal for gamma camera imaging. The low-energy 69- to 80-keV x-ray photopeak can result in attenuation artifacts and the relatively long 73-hour half-life limits the maximal dose that can be safely administered.
Monovalent Cationic Technetium-99m-Labeled Tracers
Over the years, there have been a number of 99mTc-labeled myocardial perfusion imaging agents that have been investigated as replacements for 201Tl. The most successful ones to date are the lipophilic monovalent cationic agents, 99mTc-sestamibi (sestamibi, Cardiolite) and 99mTc-tetrofosmin (tetrofosmin, Myoview), that are now widely used for clinical studies. Following an intravenous injection, the first-pass extraction fractions of sestamibi and tetrofosmin are approximately 65% and 54%, respectively, under basal resting flow conditions.5,6 Because of their lower extraction fractions compared with 201Tl, the plateau in tracer uptake observed during hyperemia occurs at lower flow rates. The effect of this “roll-off” in extraction at lower flow rates is to diminish the relative difference in tracer activities between high-flow regions and those myocardial regions subtended by a coronary stenosis, making it more difficult to detect milder stenoses.
Although these agents are members of two distinct chemical classes of compounds, isonitriles and diphosphines, respectively, they share several common properties. Unlike 201Tl, which utilizes a specific membrane-active transporter, these tracers are passively drawn across the sarcolemmal and mitochondrial membranes along a large electronegative transmembrane potential gradient, owing to their lipophilicity and positive charge.7 Once inside the mitochondria, these cationic tracers are tightly bound by the potential gradient such that there is a very slow net efflux resulting in prolonged myocardial retention times. Although ATP is not directly required for the intracellular sequestration of cationic tracers, as it is for 201Tl, the influx and retention of these tracers are energy dependent because the presence of a normal electronegative transmembrane gradient is required. With irreversible injury, the mitochondrial and sarcolemmal membranes are depolarized, and the uptake of these cationic tracers is impaired.8 Accordingly, like 201Tl, the cationic 99mTc-labeled agents can be used to assess myocardial viability.
In addition to the lower plateau in extraction mentioned, another disadvantage to both sestamibi and tetrofosmin is the problem of photon scatter from the adjacent liver that can interfere with the interpretation of myocardial perfusion defects, particularly in the inferior left ventricular wall. Accordingly, there has been renewed interest in recent years to design improved cationic 99mTc-labeled tracers that exhibit more rapid liver clearance. 99mTc-(N)(PNP5)(DBODC5)+ (DBODC5) is a lipophilic nitride that is rapidly taken up and retained by the myocardium in a manner that is mechanistically similar to sestamibi and tetrofosmin. However, studies in both rats and dogs demonstrated that DBODC5 cleared more rapidly from the liver than either of these other cationic tracers, with virtually no liver activity observed after only 1 hour.9,10 The first-pass extraction fraction of DBODC5 is intermediate to that of sestamibi and tetrofosmin.10 Although there is no improvement in the ability of DBODC5 to track myocardial blood flow at hyperemic flow rates, its more favorable biodistribution properties offer a potential advantage that warrants further investigation.
Another new lipophilic cationic tracer with improved biodistribution and very rapid liver clearance is 99mTc-[N(MPO)(PNP5)]+ (MPO). The myocardial uptake of MPO in Sprague Dawley rats was reported to be between that of sestamibi and DBODC5 over 2 hours.11 Interestingly, the heart-liver ratio of MPO at 30 minutes after injection was more than twice that of DBODC5 and approximately 4 times higher than that of sestamibi.11 With such rapid liver clearance, clinically useful images might be obtainable as early as 15 minutes post injection. At the present time, the first-pass extraction fraction studies have not been conducted using MPO.
Neutral Lipophilic Tracers
99mTc-teboroxime (teboroxime) is a member of a class of neutral lipophilic molecules known as BATOs (Boronic acid Adducts of Technetium diOxime). After intravenous injection, the initial instantaneous uptake of teboroxime is high, with a first-pass extraction fraction of approximately 90%—higher than even 201Tl.12,13 However, unlike the cationic 99mTc-labeled myocardial perfusion tracers discussed earlier that are retained in the myocardium, teboroxime exhibits rapid flow-dependent myocardial clearance in under 10 minutes. Thus, although the myocardial extraction fraction that is observed immediately after injection is very high, the rapid clearance of this tracer results in a loss of defect contrast within the first 5 minutes post injection.14 Additionally, because the myocardial clearance rate of teboroxime is flow dependent, with slower clearance from ischemic versus normally perfused zones, the differential clearance rates give the scintigraphic equivalent of “redistribution,” with an apparent filling-in of the initial perfusion defects over time, as is observed with 201Tl.15 The mechanism for such rapid clearance is that teboroxime is believed not to cross the sarcolemmal membrane into the intracellular space of the myocyte, remaining instead within the intravascular space in association with the endothelial layer.16 Furthermore, its myocardial uptake is passive, not dependent on either active transport or other energy-dependent processes. Thus, teboroxime is considered to be a pure perfusion tracer.
Another neutral lipophilic perfusion tracer that has undergone Phase III clinical testing is 99mTc-N-NOET (NOET). Like teboroxime, NOET exhibits a first-pass extraction fraction that is higher than either sestamibi or tetrofosmin, with flow-dependent differential clearance of the tracer from the myocardium.17,18 Because of the differential clearance from ischemic versus normal zones, NOET has been shown to undergo apparent redistribution like teboroxime, albeit at a slower rate.18,19 Another similarity between NOET and teboroxime involves their mechanism of localization in the myocardium. NOET is also believed to remain within the intravascular space in association with the endothelial layer.20 Because of its accessibility, NOET clearance can be affected by a host of intravascular factors. Experimental studies demonstrated that the myocardial clearance rate of NOET could be accelerated not only by increasing the flow rate but also by elevating the blood lipid concentration.16,21 Like teboroxime, the uptake and retention of NOET does not involve active or energy-dependent processes, and thus it would also be considered a pure perfusion tracer.
MODELING TRACER EXTRACTION
The tracers used for clinical imaging of myocardial blood flow are not completely extracted. For these tracers, the fraction of tracer extracted on passing through a capillary bed depends on the blood flow through the capillary bed. A model based on the work of Gosselin and Stibitz 22 provides insight into this process. The model is that of a diffusible tracer traveling through a cylindrical capillary. The tracer can diffuse outward from the blood across the capillary endothelium, but it can also diffuse back into the blood from outside the capillary endothelium. The outward and back-diffusion coefficients can be different. The extraction coefficient reflects the net loss in tracer concentration between the arterial and venous ends of the capillary. This leads to a tracer “extraction fraction” of the form:
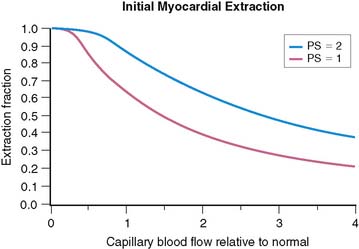
where PS is a product of capillary permeability and surface area, and b is the capillary blood flow. The relationship between blood flow and tracer extraction predicted by this model is shown graphically in Figure 1-1. The top curve with PS = 2 would represent a tracer with high first-pass extraction, such as 201Tl. The lower curve with PS = 1 would represent a tracer with lower first-pass extraction, similar to sestamibi and tetrofosmin. The term first-pass extraction is often used to characterize radionuclide tracers, but it is not often carefully defined. Since the extracted fraction of tracer is flow dependent, the first-pass extraction indicates the fraction of extracted tracer measured at baseline resting blood flow. In Figure 1-1, the first-pass extraction of the two tracers shown would be about 86% for the upper line and about 64% for the lower line.
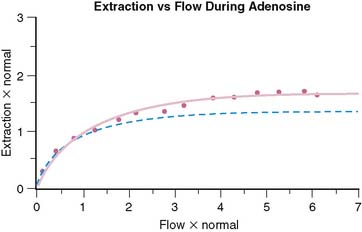
Although the equation was derived for solute exchange in a single capillary, it can be shown that the functional form remains unchanged for a generalized distribution of capillaries if the parameters are taken to represent the averages over the entire capillary distribution. The curve with the functional form shown has been ubiquitous in representing myocardial uptake as a function of myocardial blood flow. Figure 1-2 shows some experimental data of sestamibi extraction versus blood flow. The solid line of Figure 1-2 has the functional form of Equation 2. It fits the experimental data quite well if the PS coefficient is chosen empirically to best fit the data. However, if we substitute the PS coefficient that best agrees with the first-pass extraction data, it results in the dashed line of Figure 1-2 and produces a poor fit for the flow-versus-extraction curve. The dashed line predicts a more extreme reduction of tracer extraction with increasing myocardial blood than experimentally observed.
The same PS product should predict both the measured first-pass extraction coefficient and the flow-versus-extraction curve. The fact that it does not indicates that something is wrong with the model. A possible problem with the simple Gosselin and Stibitz model is that it does not account for myocardial flow regulation by opening and closing of capillary channels. Selective opening and closing of parallel capillary channels is thought to be an important mechanism to regulate capillary resistance and myocardial blood flow. This has been experimentally demonstrated.23,24 Further evidence for the role of capillary closure has been more recently found in the context of contrast echocardiography25 and for sestamibi perfusion measurements in the dog model.26
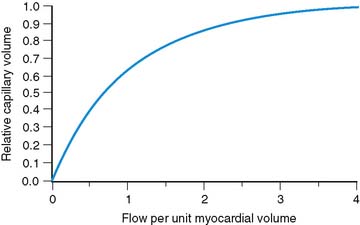
To account for the effect of variable capillary volumes, we wish to extend the basic model as follows: The first factor in Eq. 2 is replaced by F, which represents flow per unit myocardial volume. The term b in the exponential represents flow per unit of open capillary volume. We now introduce a new relationship:
Equation 3 allows for flow in the open capillaries to be different from flow per unit myocardial volume determined by the arterial supply vessels. Equation 3 further introduces the assumption that capillary blood volume decreases with decreasing flow due to capillary closure, and it increases to some maximum value when all the capillary channels are fully utilized at high flow. Figure 1-3 shows the relative capillary volume assumed by Eq. 3. This is in qualitative accord with the observations of Wu et al.23 The exact way that capillary volume changes in the course of vasoregulation is unknown. Our purpose here is limited to that of showing what effect variable capillary volume would have on tracer extraction.
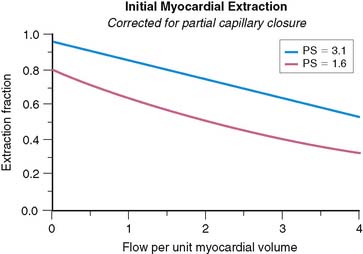
The effect of capillary closure can be seen in Figure 1-4. The curves of first-pass extraction become less blood-flow dependent. The first-pass extraction fraction at low flow is less than would be predicted by the basic model of Gosselin and Stibitz,22 and the decrease of extracted fraction with increasing blood flow is less severe. The curves of Figure 1-4 are plotted for PS = 1.6 and 3.1, which represent the values that fit the experimentally measured extraction fractions of 0.64 and 0.86 for sestamibi and 201Tl, respectively. These values, obtained from first-pass extraction data, were used to compute the myocardial uptake-versus-flow curves, and those curves are plotted with experimental data from Glover in Figure 1-5. The predicted curves fit quite well to the experimental data. Thus, by including the effect of variable capillary blood volume, we are able to simultaneously predict all the experimental data from the same PS coefficient. The modified model is self-consistent, indicating that it is a better representation of reality.