Landmark clinical trials have established the benefit of P2Y12 inhibitors in the setting of acute coronary syndrome and percutaneous coronary intervention. On February 12, 2014, the Medicines Company (Sponsor) presented efficacy and safety data regarding cangrelor to the Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee. The Sponsor sought approval for 2 indications: (1) in the setting of percutaneous coronary intervention for the reduction of thrombotic cardiovascular events (including stent thrombosis) in patients with coronary artery disease and (2) in the setting of bridging therapy in patients with acute coronary syndrome or with stents who are at increased risk for thrombotic events (such as stent thrombosis) when oral P2Y12 therapy is interrupted because of surgery. The following is a summary of the data presented to the FDA by the Sponsor, the FDA’s clinical review of cangrelor.
Landmark clinical trials have established the benefit of P2Y12 inhibitors in the setting of acute coronary syndrome (ACS) and percutaneous coronary intervention (PCI). Clopidogrel, ticagrelor, and prasugrel have thus become cornerstones in the current standard of care for antiplatelet therapy in the setting of ACS. Cangrelor is a novel P2Y12 receptor antagonist that blocks adenosine diphosphate–induced platelet activation and aggregation with unique pharmacodynamic and pharmacokinetic properties. This direct-acting intravenous drug is characterized by immediate onset. This effect is consistent and is not associated with interindividual variability as clopidogrel is because it does not require metabolic activation. Because of a short half-life (3 to 6 minutes) and a reversible binding to the P2Y12 receptor, this drug has a rapid offset that allows recovery of platelet function within 1 hour.
Cangrelor was clinically investigated in 3 clinical trials gathered in the CHAMPION program. On February 12, 2014, the Medicines Company (Parsippany, New Jersey; Sponsor) therefore presented efficacy and safety data regarding cangrelor to the Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee (CRDAC). The Sponsor sought approval for 2 indications: in the setting of PCI for the reduction of thrombotic cardiovascular events (including stent thrombosis [ST]) in patients with coronary artery disease (CAD) and in the setting of bridging therapy in patients with ACS or with stents who are at increased risk for thrombotic events (such as ST) when oral P2Y12 therapy is interrupted because of surgery. The following is a summary of the data presented to the FDA by the Sponsor, the FDA’s clinical review of cangrelor, and the recommendations made during the CRDAC meeting.
Data to Support Cangrelor Approval Presented by the Sponsor
The Sponsor used data from 3 randomized controlled trials (CHAMPION-PHOENIX, CHAMPION-PLATFORM, and CHAMPION-PCI) conducted in 25,107 patients with CAD to provide evidence on the safety of cangrelor. The trial designs are presented in Table 1 . The Sponsor presented the results of the CHAMPION-PHOENIX as the pivotal study supporting the efficacy of cangrelor in the PCI setting and the BRIDGE trial as the pivotal study supporting its use as bridge therapy.
| TRIAL Subject | Study Design | ITT Sample Size | Description | Primary objective | Primary End Point |
|---|---|---|---|---|---|
| CHAMPION-PCI (TMC-CAN- 05-02) | Prospective, randomized (1:1), double-blind, double-dummy, active-control, parallel-group Active control: clopidogrel 600 mg | 8,877 Cangrelor: 4,433 Clopidogrel: 4,444 | UA/ NSTEACS/ STEMI patients amenable to PCI | Superiority of cangrelor efficacy over clopidogrel 600 mg in patients requiring PCI | Composite of allcause mortality, MI, and IDR at 48 hours |
| CHAMPION-PLATFORM (TMC-CAN-05-03) | Prospective, randomized (1:1), double-blind, double-dummy, placebo-control, parallel-group over standard of care: including clopidogrel 600mg | 5,364 Cangrelor: 2,695 Placebo: 2,669 | UA/NSTEACS patients amenable to PCI | Superiority of cangrelor (combined with usual care) efficacy over usual care, in patients; requiring PCI | Composite of allcause mortality, MI, and IDR at 48 hours |
| CHAMPION-PHOENIX (TMC-CAN-10-01) | Prospective, randomized (1:1), double-blind, double-dummy, active-control, parallel-group Active control: clopidogrel 300 mg or 600 mg | 11,145 Cangrelor: 5,581 Clopidogrel: 5,564 | UA/NSTEACS/STEMI patients amenable to PCI | Superiority of cangrelor efficacy over clopidogrel standard of care in patients; requiring PCI | Composite of allcause mortality, MI, IDR and ST at 48 hours (MI: as per UDMI, ST: as per ARC and IPST) |
| BRIDGE (TMC-CAN-08-02) | Stage 1: Prospective, open label, dose-finding, multi-center. Stage 2: Prospective, double-blind, placebocontrolled, randomized, multi-center. | Stage 2: 207 (n=106 cangrelor; n=101 placebo) | Patients requiring bridging from oral thienopyridine Therapy prior to cardiac surgery | cangrelor ability to provide effective and consistent P2Y12 inhibition without increasing surgical bleeding compared to standard of care | Percentage of patients with PRU < 240 as determined by VerifyNow P2Y12 point of care assay measured during study drug infusion pre-surgery |
Cangrelor efficacy and safety in the setting of PCI: the CHAMPION program
The design of each trial in the CHAMPION program was presented by the Sponsor and is outlined in Figure 1 . Noted by the FDA review committee and a source of great debate among the clinical reviewers in the second part of the meeting was that although the cangrelor dose (30 μg/kg bolus and 4 μg/kg/min infusion) and duration (2 to 4 hours) were the same in the 3 studies, the comparator arm (clopidogrel) differed ( Figure 1 ). In CHAMPION-PCI, clopidogrel 600 mg was given at the time of PCI; in CHAMPION-PLATFORM, clopidogrel 600 mg was given at the end of PCI; and in CHAMPION-PHOENIX, clopidogrel dose (either 300 or 600 mg) was left to the discretion of the individual sites and was to be administered either before or immediately after PCI. At the end of the infusion, patients in the cangrelor arm received 600 mg of clopidogrel in the 3 studies. CHAMPION-PCI and CHAMPION-PLATFORM were terminated early and failed to meet their respective primary end points. Incidence of the protocol-defined primary efficacy end point, a composite of all-cause death, myocardial infarction (MI), and ischemic driven revascularization, was not significantly reduced in cangrelor-treated patients (CHAMPION-PLATFORM: 7.0% cangrelor- vs 8.0% clopidogrel-treated patients; odds ratio [OR] 0.87, 95% confidence interval [CI] 0.71 to 1.07, p = 0.1746; CHAMPION-PCI: 7.5% vs 7.1%; OR 1.05, 95% CI 0.88 to 1.24, p = 0.5929). According to the Sponsor, post hoc analysis of the PLATFORM and PCI trials suggested signs of cangrelor efficacy, including reductions in the incidence of thrombotic events, such as ST, Q-wave MI, and ischemia-driven revascularization (IDR) but not of the end point of periprocedural MI. Results using the 2007 universal definition of myocardial infarction (UDMI) demonstrated that cangrelor reduced (18% significant reduction, OR 0.82, 95% CI 0.68 to 0.99, p = 0.0374) the composite of death, UDMI, and IDR at 48 hours compared to clopidogrel 600 mg in a pooled analysis of both trials.

The CHAMPION-PHOENIX trial was therefore designed to avoid confounding periprocedural MI with evolving preprocedural MI in patients with elevated biomarkers using the UDMI. In PHOENIX, ST definition was also modified to measure events occurring during and after the procedure. Intraprocedural ST (IPST) was included in addition to Academic Research Consortium (ARC)–defined ST and was defined as any procedural new or worsened thrombus related to the stent. The results of CHAMPION-PHOENIX demonstrated that cangrelor compared to clopidogrel standard of care is effective when administered to patients undergoing PCI. At 48 hours, the CHAMPION-PHOENIX trial demonstrated that, compared to clopidogrel, cangrelor provided a significant reduction in the primary efficacy end point of all-cause mortality, MI, IDR, and ST (4.7% vs 5.9%, respectively; OR 0.79, 95% CI 0.66 to 0.93, p = 0.005), which was maintained at 30 days (6.0% vs 7.0%, respectively; OR 0.85, 95% CI 0.73 to 0.99, p = 0.035). In addition, a significant reduction in protocol-defined ST (0.8% vs 1.4%, respectively; OR 0.62, 95% CI 0.43 to 0.90, p = 0.010), and MI (3.8% vs 4.7%, respectively; OR 0.80, 95% CI 0.67 to 0.97, p = 0.022) were also observed but without a reduction in all-cause mortality (0.3% vs 0.3%, respectively; OR 1.00, 95% CI 0.52 to 1.92, p >0.999). In the pooled CHAMPION program, there was no significant increase in the primary safety outcome of Global Use of Strategies to Open Occluded Arteries (GUSTO) severe or life-threatening bleeding (OR 1.22, 95% CI 0.70 to 2.11, p = 0.48) or GUSTO moderate bleeding (OR 1.36, 95% CI 0.96 to 1.92, p = 0.08). Cangrelor was associated with an increase in GUSTO mild bleedings (OR 1.35, 95% CI 1.26 to 1.45, p <0.001) and in Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) major bleedings that were primarily driven by an increase in hematoma ≥5 cm. During the meeting, the PHOENIX investigators, represented by Dr. Robert Harrington (Stanford University, California) and Dr. Gregg W. Stone (Columbia University Medical Center, New York), rigorously defended the trial.
The unmet clinical need for a fast onset–fast offset P2Y12 inhibitor
The unmet clinical need for the use of cangelor is derived from a physician’s discomfort to preload with antiplatelet therapy before the angiography should the patient’s anatomy require urgent surgery or from the fear of inadequate antiplatelet inhibition if loaded at the time of the intervention. Compared with current strategies, cangrelor administration could decrease periprocedural ischemic events, avoid unnecessary exposure to patients who do not require PCI but do require medical treatment and allow coronary artery bypass graft (CABG) to be performed early. Furthermore, it is the only option in patients unable to take oral medications (sedation, intubation, vomiting, and shock). Because ticagrelor and prasugrel have an obligate time of gastrointestinal absorption and did not show any improvement in acute ST in their respective phase III trials, a faster onset drug may deserve its place in the novel oral P2Y12 inhibitors era.
PHOENIX design justification
Use of clopidogrel as a comparator
Clopidogrel use, dose, and timing in the control arm were particularly called into question by the FDA review. The Sponsor argued that clopidogrel remains the most widely used P2Y12 inhibitor in the United States and internationally in all clinical settings, particularly in ACS, and necessitated its use as the control. Investigators also pointed to the lack of evidence for clopidogrel dosing at the time of PHOENIX design preparation. The randomized trial did not clearly demonstrate a difference in efficacy outcomes of preloading with 600- versus 300-mg clopidogrel in ACS, rather it showed possible harm. Consistently, American and European guidelines from 2007 to 2009 did not strongly support the 600-mg loading dose and justified investigators were free to choose the clopidogrel loading dose in PHOENIX. Moreover, meta-analyses and randomized trials, such as the recent ACCOAST (Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction) trial, showed no benefit and possible harm of adenosine diphosphate (ADP) antagonist preloading. Given this equipoise, practices regarding the timing of P2Y12 inhibitors vary greatly in the United States and thereby prompted the PHOENIX design leaders to leave clopidogrel timing to the investigators’ discretion.
End point justification
On behalf of the Sponsor, Dr. Stone demonstrated that IPST is a major adverse clinical event with serious implications for patient prognosis. According to an analysis of the angiograms performed during the ACUITY and HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) studies, IPST is related to serious complications such as death and MI. In the PHOENIX trial, IPST remained a strong predictor of all adverse ischemic events at both time points (48 hours and 30 days) after controlling for potential confounders.
Cangrelor efficacy in the setting of bridge therapy: the BRIDGE trial
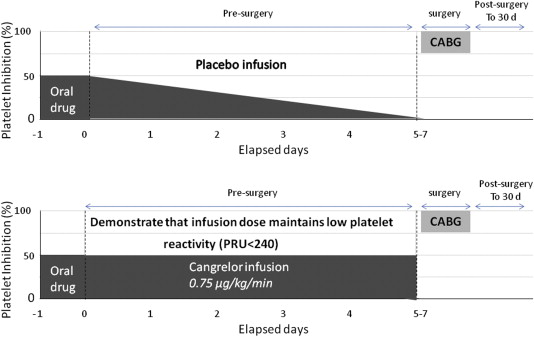
The BRIDGE trial was a pharmacodynamic study evaluating platelet reactivity of cangrelor versus placebo in ACS and/or patients with a stent who were at increased risk of thrombotic events because of discontinuation of an oral P2Y12 inhibitor before cardiac surgery ( Figure 2 ). The primary efficacy end point (percentage of patients with all samples during the infusion achieving platelet reactivity unit [PRU] <240 as determined by VerifyNow P2Y12 assay, Accumetrics, San Diego, CA) was met in 98.8% of cangrelor-treated patients compared to 19.0% of placebo-treated patients (relative risk 5.19, 95% CI 3.34 to 8.07, p <0.001). After discontinuation of cangrelor, platelet reactivity was similar for both cangrelor and placebo groups. No differences in the rates of protocol-defined excess CABG-related bleeding after discontinuation of the cangrelor infusion (primary safety end point, 11.8% vs 10.4%) and transfusion (25.5% vs 32.3%) were found between cangrelor and placebo groups, respectively. However, cangrelor use was associated with an increase in non–CABG-related GUSTO-moderate bleeding (1.9% vs 1.0%) during the 5- to 7-day bridging period. Admitting the rarity for which a labeled indication would be approved based on a phase II trial, the use of cangrelor as P2Y12 bridge therapy was sought by the Sponsor entirely on the “unmet need” platform, stating that clinicians currently have no good alternative when such a situation arises. Dr. David Schneider (University of Vermont, Vermont) presented the evidence of greater ST risk after clopidogrel discontinuation and proposed cangrelor as a logical approach to prevent these thrombotic.
A Critical Review by the FDA
Controversies in CHAMPION program, PHOENIX trial design, and data interpretation: a concern with the comparator
The data presented by the FDA review suggested that both the 600-mg loading dose and earlier timing in the clopidogrel arm were associated with better outcomes.
Clopidogrel loading dose
In PHOENIX, all patients in the cangrelor arm were to receive a 600-mg loading dose of clopidogrel at the end of the infusion by protocol, whereas the use of a 300-mg loading dose was only allowed in the clopidogrel arm. About 26% of the patients in the clopidogrel arm received 300 mg of clopidogrel. Logistic regression of the Sponsor’s primary end point in the clopidogrel arm showed that the 600-mg loading dose was the most significant predictor of outcome and led to the conclusion that 600 mg is more effective than 300 mg ( Table 2 ). The FDA statistical review implied that the imbalance in the clopidogrel loading dose between the arms of PHOENIX ultimately contributed to the favorable effect of cangrelor compared to clopidogrel.
| Odds Ratio | 95% CI | p | |
|---|---|---|---|
| 600-mg load | 0.62 | [0.47-0.82] | 0.001 |
| Age | 1.01 | [1.00-1.02] | 0.02 |
| Diabetes | 0.80 | [0.61-1.05] | 0.11 |
| Angina | 1.61 | [1.25-2.08] | 0 |
| Hours to PCI | 0.68 | [0.50-0.93] | 0.01 |
Clopidogrel timing
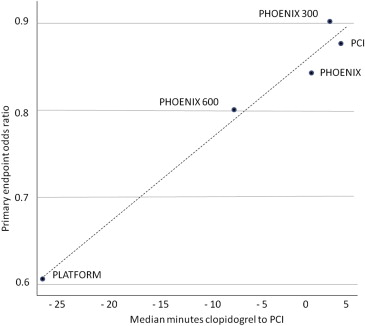
For about 32% of the PHOENIX patients, the first oral P2Y12 inhibitor was given after the completion of the PCI. In patients of the clopidogrel arm presenting with ST-elevation myocardial infarction (STEMI), the drug was administered only a few minutes (mean 6 minutes, median 4 minutes, SD 20.7 minutes) before PCI and clopidogrel was administered after angiography in 68% of the patients in this STEMI subgroup. Dr. Marciniak, medical team leader of the FDA, strongly argued the importance of clopidogrel timing in the CHAMPION program and in the PHOENIX trial as a key factor for clopidogrel efficacy. He considered that cangrelor was clearly favored by the delay in clopidogrel loading because of PHOENIX design for the following reasons. The CHAMPION trials themselves provided evidence that earlier administration of clopidogrel was better according to his different interpretation of CHAMPION. The major difference between the 2 PHOENIX predecessors was that in CHAMPION-PCI, clopidogrel was given before the PCI (but after angiography), whereas in PLATFORM, clopidogrel administration was delayed until after the PCI. His detailed analysis of the results showed that mortality and ST rates were substantially higher in the clopidogrel arm of the PLATFORM study (0.7% mortality rates at 48 hours in the clopidogrel arm of CHAMPION-PLATFORM vs 0.1% in the clopidogrel arm of CHAMPION-PCI and 0.2% in cangrelor groups of both trials, 0.6% ST rates at 48 hours in the clopidogrel arm of CHAMPION-PLATFORM vs 0.3% in the clopidogrel arm of CHAMPION PCI and 0.2% in cangrelor groups of both trials). According to Dr. Marciniak, the main lesson learned from the early CHAMPION program should have been “delaying clopidogrel is bad.” In addition, his statistical review aimed to demonstrate that differences in clopidogrel delay explain differences in the results from the 3 CHAMPION trials. Figure 3 is taken from the FDA briefing document and illustrates that the greater the delay in administering clopidogrel, the better cangrelor looked for efficacy in the CHAMPION program. Logistic regression of the PHOENIX data also confirmed earlier timing is associated with better outcomes ( Table 2 ).