Organization of Intensive Care Units and Long-Term Acute Care Hospitals
INTRODUCTION
The birth of modern day critical care is traditionally ascribed to Bjorn Ibsen, a Harvard-trained Danish anesthesiologist, who led the efforts to combat the poliomyelitis epidemic in Copenhagen in 1952.1 By relying on the manual power of volunteers to ventilate the critically ill for extended periods of time, respiratory failure-related mortality declined precipitously. From these humble beginnings, the growth of critical care medicine over the ensuing years has been exponential.
The tenets of 21st century critical care include the provision of care by professionals with diverse yet complementary experience and expertise that is delivered across the continuum of care. The nexus of critical care is the Intensive Care Unit (ICU), where critically ill patients are brought to be resuscitated and managed. It is estimated that there are in excess of 5.7 million annual ICU admissions in the United States, and the numbers continue to increase.2,3 The costs attributed to the care of the critically ill continue to increase dramatically and currently exceed $80 billion, 13% of hospital costs, 4% of the United States healthcare expenditures, and approximately 1% of the gross domestic product.4 Approximately 500,000 patients admitted to an ICU die.2 However, as a result of advances in the field, mortality is decreasing and the vast majority of critically ill patients survive. The direct result is that coordinated care is required following discharge from the ICU for these complex patients who have often sustained significant morbidity (e.g., postintensive care syndrome).5,6
STRUCTURAL ORGANIZATION
The administrative and functional organization of critical care has evolved over decades. Selected aspects of this organization are discussed in the following sections.
 THE CONTINUUM OF CRITICAL CARE
THE CONTINUUM OF CRITICAL CARE
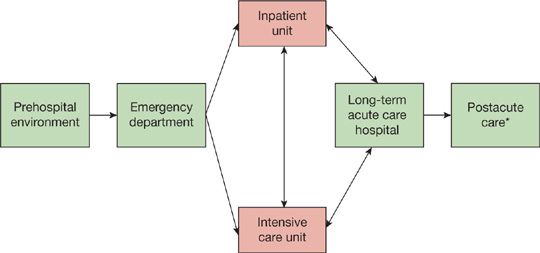
The care of the critically ill patient begins at first recognition of physiologic derangement. The opportunity to alter the patient’s course begins with the initial encounter with the healthcare system. Across the continuum of care (Fig. 152-1), this encounter may occur in the prehospital environment, outpatient clinic, or emergency department (ED). For the hospitalized patient, deterioration may occur on the medical or surgical inpatient unit. Evidence suggests that outcomes are significantly worse in patients admitted to the ICU from the general inpatient unit, in comparison to patients admitted through the ED or postoperatively.7 Once critical illness has been recognized, the tenets of care include resuscitation and stabilization and efficient and safe transport to the ICU. Traditionally, efforts to optimize care of the critically ill patient were focused on management provided in the ICU. One of the greatest advances in critical care medicine has been the recognition that the structure and processes of care in locations outside of the ICU (prehospital environment, ED, inpatient unit) are of paramount importance in optimizing care for these vulnerable patients.
Figure 152-1 The continuum of critical care. *Postacute care includes skilled nursing facility, acute rehabilitation, and home.
 THE RAPID RESPONSE TEAM
THE RAPID RESPONSE TEAM
Over the last 15 years, driven by the observation that many transfers to the ICU were either avoidable or characterized by delayed or suboptimal care,8,9 substantial growth in the use of rapid response teams (RRTs) has been observed. RRTs, also known as emergency medical teams, serve as the efferent arm of the critical care response in the hospital. Traditionally, primary care providers, including trainees inexperienced in the management of the critically ill, led the resuscitation. However, primary care providers often have competing responsibilities that may impede their ability to assess and manage the deteriorating patient in a timely fashion. Further, based on the concept that a team of experienced critical care providers tasked to respond immediately to the bedside of a deteriorating patient would result in improved outcomes, hospitals have widely adopted the RRT model as a means to improve patient safety.
The success of the RRT is dependent on the ability of the screening method to detect the deteriorating patient (“afferent” limb), as well as the staffing and experience of the efferent arm. Several strategies exist to recognize the deteriorating patient in a timely fashion. The most commonly applied strategy is the modified early warning score (MEWS), a simple and effective tool that trends vital signs and consciousness to identify patients at risk of adverse outcomes, including ICU admission and death.10 The MEWS has been validated in medical and surgical patients; an important characteristic is that it relies on serial measurements, since admission of vital signs is an insensitive marker of subsequent deterioration.11,12 Future directions aimed at optimizing the strategy include incorporation of clinical data focusing on at-risk patient populations;13 use of laboratory data and computer programming to incorporate trends in laboratory values, as well as threshold values; and use of automated information technology to improve the efficiency of the system.
Traditionally, the efferent limb or first responders were clinically available staff, who often included inexperienced trainees. The evolution in patient safety has prioritized, appropriately, that the resuscitation efforts for such vulnerable patients are led by practitioners with expertise in managing critically ill patients. It remains unclear whether outcomes differ when the team is led by a critical care certified nurse, advanced practitioner, or intensivist. Further investigation is required to address this important question, given limited resources across the continuum of care. In addition, ancillary personnel (e.g., respiratory therapists, pharmacists) are critical in facilitating delivery of time-sensitive interventions in the deteriorating patient.
Despite a sound rationale, it remains unclear whether an RRT improves patient outcomes.14 In a large multicenter trial, while more deteriorating patients were managed following implementation of an RRT, no significant decreases in the incidence of cardiac arrest, ICU admissions, or mortality were noted.15 However, in a more recent meta-analysis, the introduction of an RRT appeared to reduce the rate of non-ICU cardiac arrests.16 Furthermore, an additional potential benefit of an RRT is that its introduction may facilitate timely end-of-life discussions, preempting ICU admission when such an admission would not align with the patient’s preferences and values.17
 THE INTENSIVE CARE UNIT
THE INTENSIVE CARE UNIT
Internationally, the availability of resources to care for the critically ill varies significantly, even in developed countries.3,18 Importantly, evidence suggests that lack of ICU bed availability is associated with increased mortality.19 In the United States, there has been a rapid increase in the number of ICU beds per capita, with an estimated 5.7 million annual ICU admissions (Table 152-1).20 The growth in ICU bed availability in the United States is driven by several pressures, including the need to meet the expected demand of critical care services required to care for an aging population, and the demand resulting from high-intensity care elsewhere in the healthcare system (e.g., solid-organ transplantation, intensive chemotherapy, and high-risk surgery).18,20
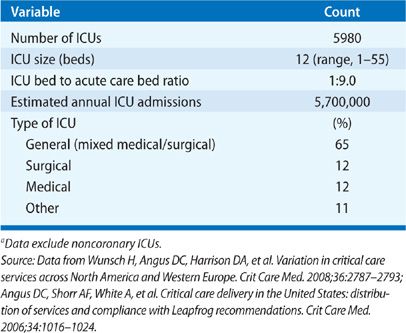
In 2007, 67,357 critical care beds were encompassed in approximately 6000 ICUs.3,20–22 This equates to 2.80 critical care beds per 10,000 persons,21,22 an increase from 2.00 beds per 10,000 persons less than a decade earlier.3 The growth in ICU beds across the United States has been transformative, as ICUs were uncommon prior to the 1970s. At the same time, the growth in ICU beds has been disproportionate at a regional level. As a result of this regional variability, the potential exists that areas with less ICU bed availability may be unable to respond to a disaster or epidemic.21
Drawn from a representative sample of hospitals across the nation, the median number of ICUs per hospital is 2, with an interquartile range of 1 to 3 and range of 1 to 8.22 In general, the number of ICUs per hospital increases in proportion to hospital size, with the smallest hospitals using a single ICU, and the largest hospitals using 3 or more ICUs.20,23 It has been estimated that approximately half of ICU beds are located in small-to-moderate size hospitals (<300 beds).20 Importantly, the optimal ratio of ICU beds to hospital beds to ensure safe, efficient, high-quality care remains unclear; furthermore, the ratio is variable from institution to institution and, within institution, from service to service (e.g., medical vs. surgical). Since 1992, the average ICU size (12 beds per ICU) has remained relatively stable, as has the proportion of hospital beds apportioned as ICU beds (8%–9%).20,23 In general, given evidence that patient admissions to an ICU, when delayed or denied due to bed availability, are at increased risk of adverse outcomes,22,24,25 a general principle is to apportion 10% to 20% of total beds as ICU beds. Institutions that provide high-intensity care (e.g., intensive chemotherapy) should allot a greater proportion of beds as ICU beds to enable provision of necessary care.
 ICU ADMISSION DIAGNOSES AND USE OF LIFE SUPPORT
ICU ADMISSION DIAGNOSES AND USE OF LIFE SUPPORT
In general, patients perceived to be at high risk for an adverse outcome are admitted to an ICU; the indication for ICU admission depends largely on the type of ICU. The ED serves as the admitting source for approximately 50% of ICU admissions. Postoperative patients account for an additional 25%, while the remaining patients are admitted from inpatient units or another hospital.20
The most common indications for ICU admission include respiratory failure, postoperative care, acute coronary syndrome and congestive heart failure, and severe sepsis.20–22 On average, the mean length of stay in the ICU is approximately 4 days, with significant variability from hospital to hospital and among ICU types (e.g., length of stay is significantly greater in burn and trauma ICUs than in general medical or surgical ICUs).20 An additional explanation for these observed differences is the considerable variation in use of intensive care, as evidenced by the variable rates of ICU admission for a resource-intensive condition associated with a very low rate of mortality—diabetic ketoacidosis.26 Further investigation is required to better understand the “appropriateness” of ICU admissions and ICU care delivery, given that an increasing proportion of our gross domestic product is allocated to such care.
Life support, including use of noninvasive and invasive mechanical ventilation for respiratory failure, vasoactive agents for cardiovascular failure, and hemodialysis for renal replacement therapy is commonly indicated. These examples of life support highlight the sophisticated technology required to guide resuscitation and support organ failure. Experience with such technology in caring for critically ill patients may explain the observed relationship between patient volume and outcomes.27,28
 GENERAL VERSUS SPECIALITY ICUS
GENERAL VERSUS SPECIALITY ICUS
Two types of ICUs within hospitals in the United States may be generally identified: general and specialty. The general ICU, or mixed medical/surgical ICU, is the care site for patients with diverse diagnoses and is the most common type of ICU (Table 152-1). Usually, these ICUs are located within smaller, community-based hospitals. In contrast, specialty ICUs are located predominantly within larger, urban hospitals. The specialty ICU (e.g., coronary care unit, dedicated medical unit, neurologic unit, cardiothoracic surgery unit) centralizes nursing and physician expertise in a single location to enable efficient, diagnosis-specific care. Interestingly, despite the rationale for specialization, the evidence does not support adopting such an organizational model in efforts to improve outcomes (e.g., mortality, length of stay).22 However, admission to a specialty ICU of a patient with a diagnosis not typically cared for by that ICU (in which the patient is considered a “boarder”) appears to be associated with increased mortality.22 Consequently, hospitals may elect specialization for a number of reasons distinct from mortality and length of stay (e.g., cost, convenience, staff and family satisfaction). However, these same hospitals should incorporate provisions for optimizing care for “boarders.”
ORGANIZATIONAL FACTORS ASSOCIATED WITH PATIENT OUTCOMES
A number of organizational factors have been identified, which, when implemented, are associated with improved outcomes. Broadly, these factors can be categorized as (1) personnel and staffing-related, and (2) processes of care (e.g., multidisciplinary patient and family-centered rounds) that streamline delivery of high-quality critical care. High-quality critical care is delivered by a dedicated critical care team,29,30 incorporating strong, collaborative physician and nursing leadership,31 operating within a “closed” unit.32–34 In an “open” model, care is provided by physicians who have competing responsibilities outside of the ICU (e.g., operating room activities or outpatient practices). In open models, critical care consultation is an option (e.g., consultation for mechanically ventilated patients). In a “closed” model, care is provided exclusively by critical care specialists.
 PERSONNEL
PERSONNEL
Multidisciplinary critical care harnesses the expertise of the entire critical care team. The personnel required to provide critical care include physicians, nurses, respiratory therapists, critical care pharmacists, nutritionists, physical and occupational therapists, speech therapists, social workers, and palliative care experts. The integrated care provided by each of these professionals is essential for optimizing care delivery.35,36 Given the ever-increasing complexity of critical care,37 each team member must contribute the necessary expertise to reduce errors, improve communication, and facilitate delivery of safe, efficient, cost-effective care.38,39 Because of the complexity of the administrative duties required to co-manage an ICU, ensuring that the medical and nursing directors are provided sufficient time to manage and lead effectively is critical.
 STAFFING
STAFFING
Physician and nursing staffing models vary across the United States; the optimal approach is unknown. Traditional physician staffing models include coverage by general practitioners (medical and surgical), elective consultation from board-certified critical care physicians (intensivists), or mandatory management by intensivists. The general practitioner model may include physicians trained in Internal Medicine (in the case of medical critical care) or hospitalists. Traditionally, ICU physician staffing is categorized as either low-intensity (staffing by general, nonintensivist practitioners with intensivist consultation) or high-intensity (mandatory intensivist consultation or primary care delivery by an intensivist).40
With few exceptions,41 a high-intensity physician staffing model, which is synonymous with a “closed” ICU is associated with improved outcomes.40 A systematic review found that high-intensity physician staffing results in lower in-hospital mortality, reduced ICU length of stay, and to a lesser degree, reduced hospital length of stay.40 While the potential benefits of high-intensity staffing are likely multifactorial, one clear benefit appears to be in use of evidence-based processes of care when care is delivered by practitioners with expertise in critical care. For example, the mortality benefit achieved when intensivists care for patients with ARDS is, in part, mediated by intensivists’ increased use of lung protective ventilation.34 Use of evidence-based protocols (see Process of Care), is one strategy to ensure delivery of evidence-based care that is applicable to ICUs, regardless of their structure and staffing.
In response to the growing costs and use of critical care, and in light of evidence that high-intensity physician staffing is associated with improved outcomes, the Leapfrog Group, a US-based alliance of employers dedicated to improving the safety and quality of healthcare and optimizing purchasing power, identified critical care staffing as one of the principal opportunities to save lives. The Group recommended provision of critical care in metropolitan areas by intensivists on-site during daytime hours and their availability to return in a time-sensitive manner during off-hours. The Group estimated that adoption of these recommendations could save >50,000 lives each year.42
A recent analysis of critical care delivery in the United States conducted by the Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) estimated that approximately 25% of ICUs provide high-intensity physician staffing.20 In addition, the majority of ICUs in the United States, in contrast to those in Western Europe, remain “open” units in which patients are managed by nonintensivists who have the option to consult intensivists.3,41,43
A legitimate threat to high-intensity staffing is the anticipated intensivist shortage, which will be exacerbated by the anticipated national nursing shortage. Preservation of a limited patient-to-nurse ratio (2:1 or less) and ICU bed-to-intensivist ratio (less than 15:1) is vital to the optimal delivery of critical care.29,43–45 Until and unless healthcare is able to avoid the pending shortage of intensivists, given the present environment, novel strategies will be necessary for optimizing critical care delivery.
Based on the relationship between volume and outcomes observed across medical and surgical critical care,27,28,46–48
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree