Occupational Asthma, Byssinosis, and Industrial Bronchitis
Inhalation of dusts, fumes, and organic substances at the workplace can cause a number of pulmonary syndromes.1–4 The lung parenchyma and airways, as well as the pleura, can be affected by inhalation of foreign substances. This chapter discusses reactions of the large airways to inhalation of toxic substances present in the workplace. Lung parenchymal and pleural reactions, as well as obliterative bronchiolitis in response to inhaled materials, are discussed elsewhere in this text. Occupational airway disease can manifest itself as chronic bronchitis with variable airway hyperreactivity (industrial bronchitis or asthma-like syndrome), or with asthma accompanied by persistent hyperreactivity of the airways (occupational asthma). Some occupational exposures can cause both industrial bronchitis and asthma whereas others cause only one or the other. Cotton dust is the most common cause of industrial bronchitis without occupational asthma. Grain dust can cause both industrial bronchitis and asthma. In this chapter, general and specific issues regarding industrial bronchitis and occupational asthma are discussed.
INDUSTRIAL BRONCHITIS
Two important causes of industrial bronchitis – byssinosis and grain dust exposure – are discussed below.
 BYSSINOSIS
BYSSINOSIS
Adverse pulmonary reactions in cotton workers have been recognized for more than 100 years. In 1831, Kay5 described chest tightness and fever that commonly occurred on Monday after workers had been off work over the weekend. It was because of this observation that the term Monday morning fever was coined. The term byssinosis was proposed by the French physician Proust6 and is derived from the Greek word meaning linen or fine flax. Over the years, as cotton mills appeared in more and more countries, the association of chronic bronchitis with cotton dust exposure was confirmed.
Epidemiology
There is no doubt that recurrent exposure to cotton dust causes acute and chronic bronchitis. In a prospective study, 16% of cotton mill workers in South Carolina developed symptoms of chronic bronchitis,7 as compared to only 1% of appropriate controls in the region. A very recent study8 of textile workers in Pakistan confirmed this finding; 16.7% of workers complained of frequent cough and 26.6% of workers had frequent phlegm production. Another recent study of cotton textile workers in China9 found that the frequency of symptoms of byssinosis increased from 7.6% at baseline to 15.3% after 15 years of working in the textile mill. In this latter study, airway flow rates decreased significantly over time in textile workers when compared to silk workers. The appearance of symptoms during work or worsening of pulmonary function tests during the work shift predicted this accelerated loss of pulmonary function. The association between the length of time working in a textile mill and the onset of symptoms was also confirmed in the study from Pakistan.
Overall textile employment has dropped over the past few years but there are still over 200,000 employed in this industry in the United States.10 These individuals are at risk for the developing symptoms due to inhalation of cotton dust. Flax and hemp workers are also at risk for developing the disease. Clinical studies suggest that approximately 65% of the general population will react significantly to de novo inhalation of components of cotton dust. Therefore, the majority of individuals who begin employment that entails the processing of cotton, flax, or hemp are at risk for developing respiratory symptoms. Why some individuals are more susceptible than others to the respiratory effects of cotton dust is unclear.
Certain jobs in the textile mill are associated with a higher risk for the development of bronchitis. Ginning, opening, or carding work carries a higher degree of risk. In addition, workers who clean out or maintain the various machines that divide up and clean the cotton are especially prone to developing symptoms. These are particularly high-risk jobs because of the high levels of cotton dust generated during the cleaning procedures. Strippers and grinders, who maintain the carding machinery that cleans and aligns the cotton, are also at risk for the development of symptoms. Indeed, in the past, byssinosis was called “strippers’ asthma.” In the recent study out of Pakistan, the job of spinning carried a higher risk for the development of respiratory symptoms than weaving.8
Clinical Presentation, Risk Factors, and Stages of Byssinosis
Shortness of breath often occurs on the day back to work at the textile mill after several days off, such as on a Monday after being off over the weekend. Overtime workers can develop more persistent symptoms. Schilling11 has graded byssinosis (Table 89-1) to allow comparison of symptomatology with physiological parameters. Using this grading system, it has been established that workers with a higher grade of symptoms tend to have a more rapid decline in pulmonary function. Risk factors for developing higher grades of byssinosis include length of employment in a cotton mill and level of cotton dust exposure. Tobacco smoking has been shown to be synergistic with exposure to cotton dust in producing chronic bronchitis. There is evidence that exposure to cotton dust without cigarette smoking causes chronic pulmonary disability, approximately 7% of exposed individuals will develop irreversible airway obstruction that cannot be explained by smoking.10 The degree of decrease in flow rates in function tests before and after work predicts chronic effects.9
TABLE 89-1 Clinical Grading of Byssinosis as Proposed by Schilling

Pulmonary Function Test Abnormalities
Characteristically,10 byssinosis is associated with a reduction in the forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) on the day of return to work after an absence. The degree of reduction in these parameters increases over the workday. This change is generally more severe on the first day of work after an absence and attenuates on subsequent continuous workdays. The mechanism by which this developed tolerance occurs is unknown although there are studies demonstrating that the inflammatory effects of endotoxin, the purported causative agent of byssinosis, attenuate with repeat exposure.
Pathology and Pathogenesis of Byssinosis
The histopathology of byssinosis12 is similar to that of the bronchitis that is induced by tobacco smoke—with hyperplasia of mucus glands and infiltration of the bronchi with polymorphonuclear neutrophils. Several animal studies have demonstrated that different components of cotton dust can recruit neutrophils into bronchi. In addition, components of cotton dust can also stimulate resident pulmonary cells, such as mast cells and macrophages, to release molecules that attract neutrophils.
There is now a large amount of information that points to a lipopolysaccharide (endotoxin) produced by bacterial contaminants of cotton as the causative agent of byssinosis. The evidence for this is listed in Table 89-2. The most compelling study that examines this issue was presented by Castellan et al.,13 who demonstrated that ambient concentrations of endotoxin in a simulated carding room correlated with reduction in airway flow rates in a time frame similar to that which occurs after exposure to cotton dust in the workplace. An interesting related finding is that byssinosis is less prevalent in Australia, probably because of the lower level of endotoxin in cotton grown in this drier climate.10 The acquired tolerance during the work week displayed by patients with byssinosis can be simulated by administration of multiple aerosols of endotoxin in animals.10,13
Treatment and Prevention
The most important interventions for byssinosis are removal of the symptomatic individual from the offending work environment and reduction in cotton dust as a preventative measure.10 Screening pulmonary function testing at the workplace is important to identify susceptible individuals who exhibit airflow abnormalities. In addition, since the 1970s, measures have been taken in developed countries to control cotton dust levels in textile mills. One measure has been to steam-clean cotton while it is still in the bale. In 1970, Burlington Industries began a program for dust control and annual medical surveillance. With this program, the incidence of symptoms of byssinosis dropped from 4.5% in 1970 to 0.6% in 1979. In addition, the number of employees who had a significant decrease in FEV1 over the work shift decreased from 18% in 1971 to 3.5% in 1979. Similar measures have been taken in other textile plants, with good success in controlling byssinosis. Unfortunately these measures have not been implemented worldwide, and there remains a significant prevalence of byssinosis outside of the United States.
 GRAIN DUST–INDUCED INDUSTRIAL BRONCHITIS
GRAIN DUST–INDUCED INDUSTRIAL BRONCHITIS
Exposure to grain dust can also result in the development of chronic bronchitis.14 This can occur in conjunction with development of organic dust toxic syndrome, a term recently proposed to describe a noninfectious febrile illness associated with chills, malaise, myalgia, dry cough, dyspnea, headache, and nausea that occurs after organic dust exposure. Not only grain workers, but farmers are susceptible to development of this syndrome. Between 4% and 11% of grain workers show a reduction in FEV1 of 10% or greater over the work shift. This reduction in flow rates is directly related to the amount of dust in the air. Studies have suggested that the component of grain dust responsible for causing airway symptoms is also endotoxin, the apparent active component of cotton dust. Grain dust extract, possibly its endotoxin contaminant, can activate complement, and this may be a mechanism by which grain dust induces inflammation in bronchi. However, in contrast to cotton dust, grain dust can, in sensitive individuals, also precipitate an acute drop in airway flow rates14 rather than the slow reduction in flow rates similar to that precipitated by cotton dust. This finding suggests that airway reactions to grain dust may be heterogeneous. Grain dust also tends to produce skin abnormalities in affected individuals, in contrast to cotton dust, which generally does not cause skin reactions.14
OCCUPATIONAL ASTHMA
Below are considered the definition of occupational asthma, its risk factors, clinical presentation, mechanisms, diagnosis, and management. In addition, multiple specific examples of occupational asthma are discussed.
 DEFINITION AND LIST OF OFFENDING AGENTS
DEFINITION AND LIST OF OFFENDING AGENTS
Occupational asthma is characterized by variable airway obstruction resulting from exposure to ambient dusts, vapors, gases, or fumes incidentally present at a workplace.15 Bronchial hyperresponsiveness to nonspecific agents, such as methacholine or histamine, is usually present in these patients. An important differentiation is between the true occupational asthma caused de novo by the offending agent and underlying asthma exacerbated by the offending agent (work exacerbated asthma [WEA]). The 1995 American College of Chest Physicians (ACCP)4 consensus statement for the diagnosis of occupational asthma includes several criteria that can be used for the definitive or probable diagnosis of the disease (Table 89-3). A more recent (2007)16 consensus statement further defines steps in diagnosis and management of work-related asthma (occupational asthma and work exacerbated asthma) (Table 89-4).
FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow.
Source: Data from Tarlo SM, Balmes J, Balkissoon R, et al. Diagnosis and management of work-related asthma: American College of Chest Physicians Consensus Statement. Chest. 2008;134(3 Suppl);1S–41S.
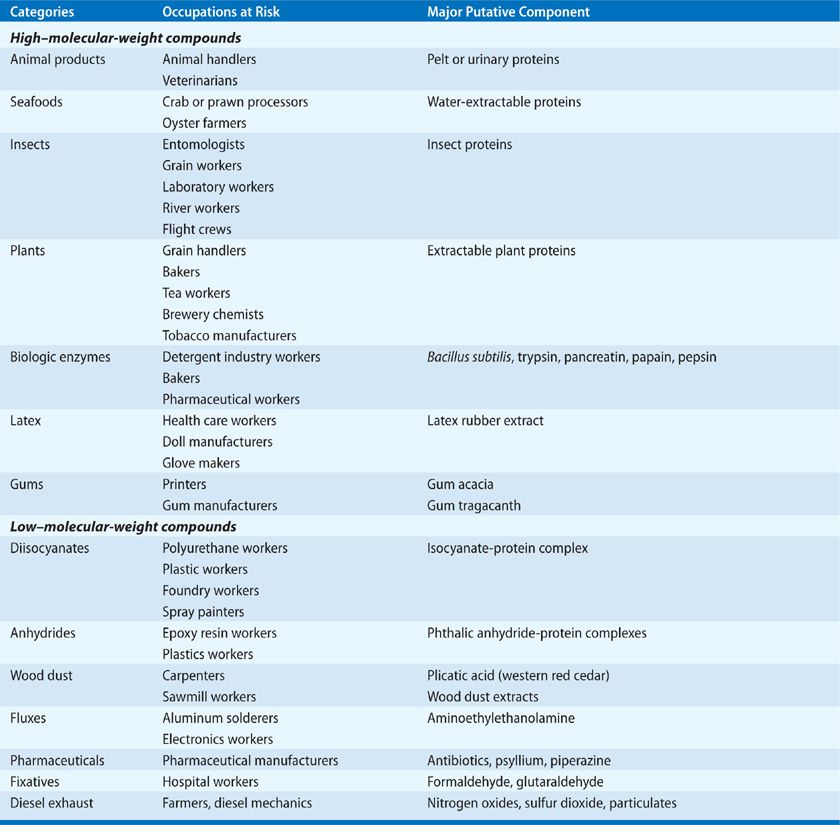
Several hundred agents17 have been reported to cause occupational asthma. Agents that have been associated with induction of occupational asthma can be conveniently grouped into categories of high- and low–molecular-weight (MW) compounds (Table 89-5). All of these agents tend to sensitize the individual, so that low ambient concentrations of the substance can ultimately cause significant bronchoconstriction. In addition, certain agents can cause direct irritant-related bronchoconstriction and airway hyperreactivity. Because the number of agents associated with occupational asthma is large and expanding, several websites have been established to allow health-care providers to obtain up-to-date information on agents that induce occupational disorders. One of these is www.occupationalasthma.com
 RISK FACTORS
RISK FACTORS
Atopy appears to be the major risk factor for developing occupational asthma, particularly when the inciting agent is a high-MW compound that induces an antibody response. Family or personal history of atopy appears to put the subject at risk. Because low-MW agents can induce asthma through nonallergic as well as allergic mechanisms, atopy may not be as important with these agents. Smoking is also a risk factor for the development of occupational asthma, particularly in workers exposed to platinum salts and anhydride compounds.3 There have been several studies documenting that workers who smoke have a higher incidence of asthmatic reactions to specific airborne agents, possibly due to overall higher immunoglobulin E (IgE) levels in smokers as compared with nonsmokers.3 There may also be genetic factors that predispose to occupational asthma. Major histocompatibility complex class II proteins are important for development of occupational asthma due to acid anhydrides, diisocyanates, western red cedar, platinum salts, latex, and animal proteins.18 Certain glutathione S-transferase and N-acetyltransferase genotypes also predict development of occupational asthma in certain settings.18
 CLINICAL PRESENTATIONS
CLINICAL PRESENTATIONS
Occupational asthma presents in a similar manner as other forms of asthma. If the physician does not maintain a high index of suspicion, symptoms will be treated but the inciting agent will not be identified. Two general forms of occupational asthma have been identified: one in which symptoms occur after a period of exposure to the inciting agent (occupational asthma with latency) and another in which symptoms develop immediately with exposure to the agent (occupational asthma without latency or irritant-induced asthma or reactive airway dysfunction syndrome). In general, the former syndrome is associated with a true allergic reaction to the offending agent while the latter is generally mediated nonimmunologically.
Occupational Asthma with Latency
Most commonly patients who develop occupational asthma do so after a period of exposure to the inciting agent. Agents that induce this sort of pattern include high- and low-MW molecules. Individuals are usually exposed to the agent for weeks to months before developing symptoms. With the appearance of symptoms, nonspecific airway hyperreactivity, determined by methacholine or histamine challenge, is present. Also with appearance of symptoms, the individual develops airway reactivity to low ambient concentrations of the offending agent. Therefore, exposure to very low concentrations of the material in the workplace precipitates severe bronchoconstriction in these patients. Controlled simulated exposure with the offending agent will elicit bronchoconstriction in patients with this syndrome, especially when asthma is due to a high-MW molecule.16,19
Occupational Asthma without Latency (Irritant-Induced Asthma, Reactive Airway Dysfunction Syndrome)
This syndrome20 is less common but can be devastating. Symptoms develop within hours of exposure. Pathological changes are generally similar to those occurring in the syndrome of occupational asthma with latency, although epithelial changes such as desquamation and subepithelial fibrosis may be more prominent. Agents that commonly cause this syndrome are irritant gases or fumes such as chlorine or ammonia (see Chapter 90). In addition, certain agents such as acid anhydrides and isocyanates can cause occupational asthma with and without latency. Reactive airway dysfunction syndrome is a form of occupational asthma without latency. The criteria for diagnosis of this syndrome are listed in Table 89-6.



