Introduction and historical perspective
Obesity is a highly prevalent and complex chronic disease that results from the accumulation of excessive adipose tissue. Hippocrates (ca. 460–370 BC) was one of the first to warn of the dangers of obesity when he wrote, “It is very injurious to health to take in more food than the constitution will bear, when, at the same time one uses no exercise to carry off this excess”. In modern times, the continued rise in obesity prevalence occurring throughout the world2 threatens to reverse gains made in prolonging life expectancy3 and reducing the morbidity from coronary disease and other chronic health problems.4 On a very simplistic level, the rapid rise of obesity in modern society can be thought of as the inevitable consequence of placing a population preselected for efficient fat storage into a sedentary environment of caloric overabundance. However, our understanding of how and why obesity develops is still incomplete, partly due to the complex interplay of socio-economic, behavioral, cultural, metabolic and genetic factors that contribute to the obesity epidemic.5,6 Developing a clear understanding of these factors, reversing the rise in obesity prevalence and treating the multitude of associated obesity-related complications represents a challenge of considerable magnitude for current and future generations.
Definitions of overweight and obesity in adults have varied over time.7 Ideally, a health-oriented definition of obesity would be based on the amount of excess body fat that correlates with the weight-responsive health risk in a given individual.8 Body mass index (BMI), defined as weight in kilograms divided by height in meters squared (kg/m2), is an easily obtained measure that is now widely used, as it has a high correlation with excess body fat or adiposity. However, BMI is not a measure of body fat and does not convey information on regional fat distribution. The latter is important, as it is now well established that central or visceral fat deposition is a major independent determinant of the metabolic and cardiovascular risk associated with increased body fat mass.9–12 Recent evidence-based guidelines therefore recommend the use of both BMI and waist circumference in the assessment of overweight or obese patients.5,6 Table 18.1 summarizes the current classification of overweight and obesity by BMI, waist circumference and associated disease risk. It should be noted that the waist circumference cut-off levels of 88 cm for women and 102 cm for men were primarily chosen because they correspond to BMI levels of 30 kg/m2 and not because validation studies have demonstrated these cut-off points to have greater discriminatory power in predicting obesity-related events.5 In fact, lower, ethnic specific cut-off levels to predict disease risk have been recently proposed, particularly for Asian and South Asian populations, but these are preliminary and require further study and validation.5
Table 18.1 Classification of overweight and obesity by body mass index (BMI), waist circumference, and associated disease risk6
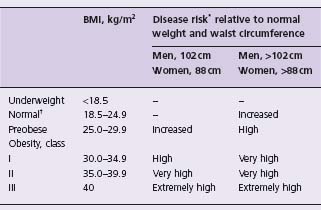
* Disease risk for type 2 diabetes, hypertension and cardiovascular disease.
– indicates that no risk at these levels of BMI was assigned.
† Increased waist circumference can also be a marker for increased risk even in persons of normal weight.
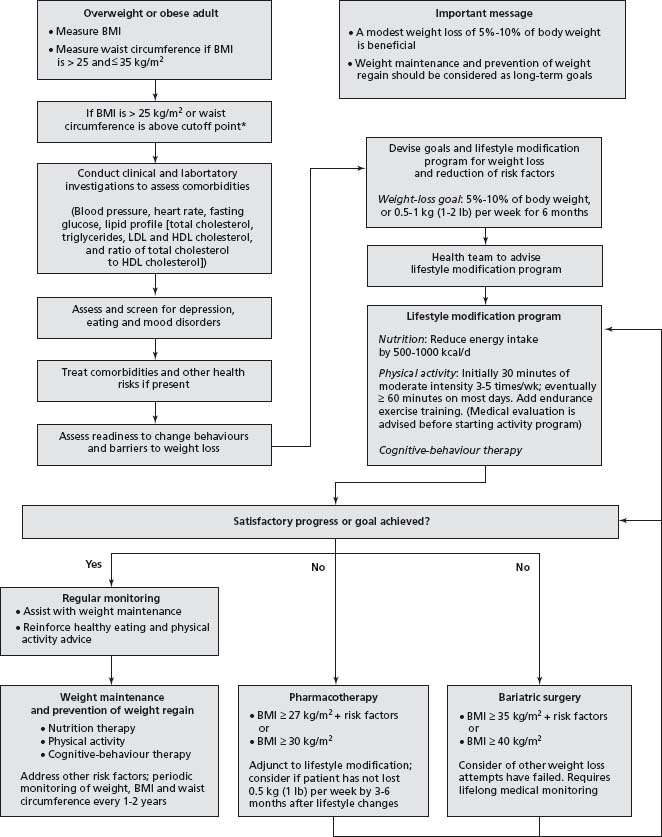
Although there are benefits to the identification of cut-off points for monitoring overweight and obesity, it is important to realize that (as for other risk factors) health risks associated with increasing weight are part of a continuum, and individuals with BMI <25kg/m2 can have substantial weight-associated health problems (for example, impaired glucose tolerance, hypertension), whereas others may have no identifiable health problems at BMI levels signifi-cantly greater than 25 kg/m2. Individualized assessment of risk status and conditions associated with obesity must therefore form an integral part of patient assessment, before deciding on the potential benefits to be derived from weight management in an individual patient (Fig. 18.1).5,6
Figure 18.1 Algorithm for the assessment and treatment of obesity. (Adapted from the Canadian Clinical Practice Guidelines on the Management and Prevention of Obesity.5)
Incidence, natural history and prognosis
The worldwide prevalence of obesity is currently estimated at over 300 million individuals, with an additional 800 million people classified as overweight.2 Approximately 40–70% of the adult population in industrialized countries and a substantial proportion in developing countries are now considered overweight or obese.13 Particularly concerning is the disproportionately rapid rise in the prevalence of severe (Class III) obesity, currently estimated at 5% in the US.14,15 Severely obese individuals are at much higher risk of developing obesity-related complications and premature death and use substantially more healthcare resources than those with milder degrees of obesity.16
Monogenic obesity syndromes such as congenital leptin deficiency present with early-onset severe childhood obesity but are extremely rare.17 Most individuals have a polygenic and environmental cause for excess adipose tissue and develop obesity later in life, with the majority of women experiencing their largest amount of weight gain after pregnancy and men, after activity wanes in the second and third decades of life.18 Slow, progressive weight gain is the general rule; due to the highly precise nature of body weight regulation, only a small positive daily caloric intake is needed to cause substantial weight gain over a long time period. For example, a weight gain of 9.1 kg (20 lbs) over a period of 30 years corresponds to an excess intake of only 0.3% of ingested calories.19 Therefore, it is remarkably easy to gain weight and modest weight gain over the short term often remains unrecognized.
BOX 18.1 Health hazards associated with obesity
- Type 2 diabetes, prediabetes (impaired glucose tolerance, impaired fasting glucose)
- Hypertension
- Dyslipidemia
- Metabolic syndrome
- Cardiovascular disease: coronary artery disease, stroke, congestive heart failure, atrial fibrillation
- Non-alcoholic fatty liver disease
- Respiratory disease: sleep apnea, obesity-hypoventilation syndrome, restrictive lung disease
- Cancers, particularly gastrointestinal and reproductive
- Osteoarthritis
- Cholelithiasis
- Gastrointestinal reflux disease
- Infertility
- Venous stasis
- Frequent infections, including cellulitis and intertrigo
- Urinary incontinence
- Idiopathic intracranial hypertension
- Psychosocial: psychiatric disease (with severe obesity), reduced employment, increased absenteeism, limited mobility, discrimination, reduced quality of life
Obesity leads to a number of health hazards (Box 18.1), many of which develop only after decades spent in the obese state. The most significant risk associated with obesity is type 2 diabetes. Compared to individuals with a normal BMI level, the 10-year risk of developing diabetes is increased approximately 20-fold in individuals with a BMI of ≥35kg/ m2.20 Weight gain has also been associated with a significant increase in coronary risk. A weight increase of 15 kg after age 21 is associated with an increased coronary risk of 83% in women and 46% in men.21 Ultimately, obesity diminishes life expectancy: a 40-year-old obese non-smoker can expect to die 6–7 years prior to a non-obese counterpart.22 A meta-analysis of 57 observational studies involving nearly 900 000 participants demonstrated that mortality is lowest with BMI levels between 22.5 –25kg/m2. Above this range, each 5kg/m2 increment in BMI was associated with a 30% higher overall mortality (HR 1.29; 95% CI, 1.27–1.32) and significant increases in cause-specific mortality (vascular, diabetic, renal, hepatic, neoplastic, and respiratory).23
Major factors associated with an increased risk of weight gain include low birth weight,24 absence of history of breast feeding,25 obesity in later childhood and adolescence,26 pregnancy,27 sedentary lifestyle,28 obesity within a family member or close social contact,26,29 smoking cessation,30 and poor dietary restraint.31
The mechanisms underlying the relationship between obesity and hypertension remain poorly understood. Several theories implicate increased sympathetic activity, sodium and volume retention, renal abnormalities, insulin resistance and, more recently, hyperleptinemia.32,33
Obesity predisposes towards type 2 diabetes primarily by increasing insulin resistance.34 Hormones secreted by adipocytes (adipokines), such as tumor necrosis factor alpha (TNF-alpha35), are important promoters of inflamma-tion and insulin resistance. Elevated free fatty acid levels, which are commonly found in the obese state, impair insulin secretion and enhance insulin resistance.36
Achieving weight loss
Prior to or in conjunction with developing a treatment plan, one must assess and treat co-morbidities and determine barriers to weight loss including identifying readiness to change (see Fig. 18.1). In-depth explanation of these issues is beyond the scope of this chapter and the reader is referred to more detailed discussions.5 Successful weight loss often requires the combination of multiple interventions and strategies, including diet, physical activity, behavior modification, pharmacotherapy and surgery (see Fig. 18.1). Because obesity is a chronic condition, all treatment, including phar-macotherapy, should be initiated with the expectation that, if successful, it will be continued over the long term.5,6
Goals of treatment
The primary goals of obesity treatment are to reduce body weight, maintain weight loss over the long term, improve obesity-related co-morbidity and increase quality of life.5,6 Weight loss should be recommended for all patients with excess weight and obesity.5,6 The initial recommended minimal weight loss target of 5–10% of baseline should be achieved gradually through a 500–1000 kcal/d (2092–4184kJ/day) deficit, which should lower weight by 1–2 lbs per week in most individuals.6 This degree of weight loss may appear trivial but is associated with improvements in obesity-related co-morbidities, as detailed below. The amount of weight loss and rate at which weight loss is achieved may be slower in patients with type 2 diabetes compared to non-diabetics.6
Lifestyle modification
With regard to dietary therapy, a review of 34 RCTs6 concluded that an average weight loss of 8% can be obtained over six months with a 500–1000 kcal/day deficit diet, and that this weight loss is associated with a decrease in abdominal fat (Class I, Level B). An updated review of 41 randomized controlled trials (RCTs) of at least one year in duration found that counseling to reduce caloric intake and increase physical activity reduced weight by approximately 3–5kg (Class I, Level B).37 Treatment is more successful with intensive therapy (defined as follow-up more frequently than monthly) and with multimodal interventions (diet, exercise and/or behavioral therapy).37 Dietary therapy generally produces the greatest amount of weight loss within the first year, with 50% of the weight initially lost regained within the first three years (Class I, Level B).38
Very low-calorie diets (VLCDs), generally involving the use of protein and dietary supplements and a caloric intake of 800 kcal/d or less, can produce greater initial weight losses than LCDs, but long-term (> 1 year) weight loss appears to be only marginally higher than LCDs (Class I, Level B).39 The most important element of dietary therapy appears to be caloric restriction. Varying dietary macronutrient composition (e.g. low carbohydrate, low fat or low glycemic index) does not result in materially greater amounts of weight loss (Class I, Level B).40–42 Importantly, unless accompanied by physical activity, weight loss through dietary modification alone does not appear associated with an improvement in cardiorespiratory fitness as measured by maximum oxygen consumption.6
Physical activity alone does not appear to materially reduce weight but improves cardiorespiratory fitness, reduces abdominal fat and, in conjunction with dietary modi-fication, assists with long-term weight maintenance (Class I, Level B).6,43 A standard recommendation is that individuals engage in at least 30 minutes of moderate intensity physical activity 5–7 days per week. Individuals who achieve over 200 minutes of activity per week achieve greater weight loss than individuals performing 150 minutes per week or less.5,44 There is also emerging evidence that accumulated, shorter bouts of activity throughout the day achieve the same health benefits as a single, longer activity session (Class I, Level B).45
There also appears to be additional value in behavioral therapy for selected patients as an adjunct to diet and exercise therapy.6,37 Standard behavior modification techniques include self-monitoring and goal setting, modifying eating behaviors (e.g. slowing the rate of eating, controling where eating occurs, delaying gratification), stimulus control and reinforcement management.5 In a meta-analysis of six short-term (< 1 year) RCTs involving 467 overweight or obese adults, behavioral therapy resulted in 4.7 kg (95% confidence interval (CI) 4.5–4.9) greater weight loss compared to diet and exercise therapy alone (Class I, Level B).46 There is a need for longer-term studies of behavioral therapy to confirm these findings.
Hypertension
A meta-analysis of 25 RCTs enrolling 4874 patients and with a mean follow-up duration of 66 weeks demonstrated that a net weight reduction of 5.1 kg (95% CI 4.3–6.0), by means of energy restriction, increased physical activity or both, reduced systolic blood pressure by 4.4 mmHg (95% CI 3.0–5.9) and diastolic blood pressure by 3.6 mmHg (95% CI 2.3–4.9). Blood pressure reductions were 1.1 mmHg (95% CI 0.7–1.4) systolic and 0.9 mmHg (95% CI 0.6–1.3) diastolic when expressed per kilogram of weight loss (Class 1, Level B).47 A subsequent meta-analysis of 14 observational and randomized controlled studies with a duration of follow-up of two years or greater demonstrated that the blood pressure reductions associated with weight loss over the long term were about half that predicted by shorter term studies (Class 1, Level B).48 In this analysis, for every 10 kg of weight loss, diastolic blood pressure was reduced by 4.6 mmHg and systolic blood pressure by 6.0 mmHg.
Which antihypertensive agent is best suited for the obese hypertensive patient? Current hypertension guidelines do not make specific recommendations on this issue, likely because of the lack of availability of high-quality data.49 A 16-week study of 171 obese patients treated with felodipine/ramipril, verapamil/trandolapril or metoprolol/ hydrocholorthiazide and randomized to sibutramine versus placebo found no significant difference in blood pressure control amongst the study arms (Class I, Level B).50 Beta-blocker/thiazide therapy, however, was associated with a 2–3 kg incremental weight gain compared to the other antihypertensive combinations, which was likely due to the beta-blocker component.51 In observational studies and post hoc/secondary analyses of RCTs, beta-blocker therapy and thiazide diuretics have been associated with an increased risk of type 2 diabetes whereas angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers have been associated with a decreased risk (Class I, Level B).52,53 However, a three-year randomized trial of 5269 patients at high-risk for type 2 diabetes found no significant effect of ramipril on the incidence of type 2 diabetes (hazard ratio (HR) 0.91, 95% CI 0.81–1.03) (Class I, Level A), although there was a signifi-cant increase in the proportion of individuals with impaired glucose tolerance (IGT) or increased fasting glucose (IFG) who regressed to normoglycemia.54 These accumulated data, although not definitive, suggest that ACE inhibitor or angiotensin receptor blockers may be preferentially prescribed to control hypertension in obese patients at high risk for developing type 2 diabetes.
Diabetes
A large number of studies have documented the benefits of even moderate (5–10%) weight loss in improving metabolic control in diabetic patients.5,6 However, the impact of weight reduction on the long-term incidence of diabetic complications and survival has not been demonstrated. Improvement in metabolic control depends more on the amount of weight loss, rather than on the method by which this is achieved. A 5 kg weight loss should decrease fasting plasma glucose levels in a diabetic individual by 1 mM or 18mg/dL.21 This is of a magnitude similar to that provided by many of the oral hypoglycemic agents. Although phar-macologic or surgical weight loss does not appear to improve glucose control beyond that achieved by lifestyle changes alone, both the degree of loss and the number of individuals achieving and maintaining weight loss are generally higher when lifestyle changes are combined with medication or surgery.5,6
Evidence from a meta-analysis of 10 randomized prospective trials indicates that lifestyle modification including modest weight reduction will markedly reduce the incidence of type 2 diabetes in high-risk individuals (HR 0.51, 95% CI 0.44 –0.60) (Class I, Level A).55 The best known of these trials, the Diabetes Prevention Program (DPP), employed a lifestyle intervention which aimed to reduce body weight by 7% and increase physical activity to at least 150 minutes per week in non-diabetic persons (mean BMI 34) with elevated fasting and postload plasma glucose concentrations. Over 2.8 years, this lifestyle intervention reduced the incidence by 58% compared with controls and was also significantly more effective than metformin in reducing diabetes incidence.56 A large, 11.5-year RCT of 5145 patients with type 2 diabetes using the lifestyle intervention employed in the DPP trial is currently under way.57 This study, known as the Look AHEAD (Action for Health in Diabetes) trial, is the first study to examine the impact of intentional weight loss through lifestyle modification on cardiovascular morbidity and mortality.
In contrast to metformin and acarbose, other antidiabetic medications, including sulfonylureas, thiazolidinediones and insulin, promote weight gain. Weight gain in patients with both type 1 and type 2 diabetes is associated with an increase in blood pressure and deterioration of metabolic control.58,59 In a secondary analysis of the UK Prospective Diabetes Study, metformin was more effective than sulfo-nylureas or insulin in reducing diabetes-related endpoints and all-cause mortality.60 Furthermore, metformin appears cost-saving when used as first-line pharmacologic therapy in overweight type 2 diabetics.61
Dyslipidemia
Lipid abnormalities in overweight and obese individuals are typically characterized by high triglycerides, increased small low-density lipoprotein (LDL) particles and low high-density lipoprotein (HDL) cholesterol levels.5,62 In the presence of abdominal obesity, high serum triglycerides are commonly associated with a clustering of metabolic risk factors known as the metabolic syndrome (atherogenic lipoprotein phenotype, hypertension, insulin resistance, glucose intolerance, prothrombotic and proinflammatory states). Thus, in obese patients, elevated serum triglycer-ides are a marker for increased cardiovascular risk. The US National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP III) therefore recognizes the metabolic syndrome as a secondary target of risk reduction therapy, after the primary target, which is LDL cholesterol.62
Numerous studies document the short-and medium-term benefits associated with lifestyle modification on blood lipid levels.63 Current evidence-based guidelines thus recommend weight reduction and increased physical activity as first-line therapies for all lipid and non-lipid risk factors associated with the metabolic syndrome.5
Pharmacotherapy
Antiobesity drug therapy can augment diet, physical activity and behavioral therapy to reduce weight and improve obesity-related co-morbidity5,6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


