Chapter 28 Nuclear Imaging in Revascularized Patients with Coronary Artery Disease
BACKGROUND
Single-photon emission tomography (SPECT) continues to be the mainstay of scintigraphy. Many technical improvements have been made over last decade, allowing shortening of acquisition protocols and attenuation correction. Hybrid SPECT/CT and positron emission tomography PET/CT systems have grown throughout the United States, opening an opportunity for more accurate noninvasive myocardial perfusion imaging1 PET unlike standard SPECT allows assessment of true coronary flow reserve.1 However, vasodilator stress nitrogen (N)-13-ammonia or oxygen (O)-15-water requires on-site isotope production and additional capital investment for a cyclotron that may be prohibitively expensive for most PET centers. Rubidium-82 PET imaging is a more practical and financially feasible alternative to SPECT imaging, with advantages of higher image quality and shorter patient time commitment.2
EVALUATION AFTER CORONARY ARTERY BYPASS GRAFT
Abnormalities observed on stress nuclear myocardial perfusion imaging (MPI) in patients who have undergone CABG will reflect a complex constellation of underlying pathophysiology that involves variable time courses. Early after CABG, abnormal perfusion may be related to early graft closure, which occurs in 12% to 20% within the first year.3 However, it may also reflect myocardium supplied by diseased vessels that were technically unable to be grafted due to anatomic limitations. Alternatively, early MPI ischemia may be the result of coronary lesions proximal to patent grafts that compromise retrograde flow. When this involves the left anterior descending (LAD), a pattern of basal ischemia involving the anterior and lateral walls may be seen. Therefore, analysis and interpretation of MPI in revascularized patients should be done with knowledge of the coronary anatomy and operative results on hand.
Late after CABG, MPI abnormalities can reflect development of intercurrent venous graft closure, which is 2% to 4% per graft per year for years 1 through 5 and reaches 50% by 5 years.3 Late ischemia on MPI can also reflect progression of native CAD.
Detection of Graft Disease
Several studies have evaluated the ability of nuclear MPI to detect graft disease, although their value is limited by relativity small sample size and older planar technology.4,5 Combining these studies, the sensitivity for detecting occluded grafts was 19/22 (86%) grafts, with a specificity of 56/64 (88%). MPI SPECT imaging has shown similar results. In a series of 109 patients undergoing adenosine SPECT MPI nearly 7 years after CABG, the sensitivity of detecting graft disease was 96%. The specificity was only 61%, but the large majority of “false positives” were explained by significant lesions in nonrevascularized native CAD or prior myocardial infarction with fixed defects.6 Sensitivity of 80% to 84% with specificity of 80% to 90% has been reported in several smaller studies.7,8 Localization of graft-site occlusion using stress nuclear MPI is quite accurate, with individual coronary territory sensitivities of 92%, 82%, and 75%, respectively, for the LAD, right coronary (RCA), and left circumflex (LCX).8 The corresponding specificities were 91%, 90%, and 75%. The lower accuracy for the LCX territory is consistent with MPI data for native CAD and probably reflects the smaller myocardial territory and overlapping distributions with both RCA and LAD.
Anatomy Versus Physiology Considerations
Because stress nuclear MPI will reflect the hemodynamic significance of a coronary or graft lesion that may not correspond to conventional paradigms of angiographically significant lesions, descriptions of sensitivity and specificity for stress MPI based on such anatomic standards is limited. For example, Salm and colleagues found that 50% of grafts with a greater than 50% diameter stenosis had normal perfusion.9 However, when graft lesions were assessed hemodynamically by Doppler coronary flow reserve, all of the grafts with depressed flow reserve had abnormal stress MPI, and 79% of grafts with normal flow reserve had normal MPI. Similar findings were reported by the same group using coronary flow reserve measured by cardiac MRI.10 Zafrir and colleagues identified that in patients with left internal mammary artery (LIMA) to LAD anastomosis, myocardial ischemia by MPI can occur without angiographic luminal stenosis, concluding that flow-limiting factors like mismatch between LAD and LIMA diameters at the anastomosis take place.11
Assessment Early After CABG (2 to 3 Years)
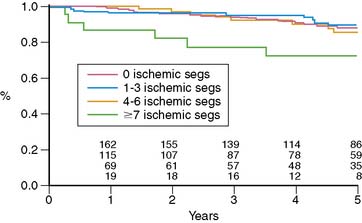
Miller and colleagues evaluated the prognostic value of stress thallium-201 MPI SPECT performed within 2 years of CABG (mean, 11 months) and followed for 5.8 years.12 They found that the only significant multivariate predictors of cardiac death or MI were the exercise angina score and the number of abnormal segments on stress MPI, which reflects the extent of ischemia plus scar. Five-year event-free survival was 93% without angina and either normal MPI or a small postexercise perfusion defect (less than 3 abnormal segments of 14 total segments), compared to 71% for patients with angina and a moderate to large defect and 83% for patients with either of these adverse prognostic factors. Looking at the prognostic impact of reversible defects, indicating jeopardized viable myocardium, there was a threshold effect where patients with more than 7 ischemic segments had a worse outcome (72% 5-year death/MI event-free rate) compared to patients with 0 to 6 reversible segments (85% to 89% 5-year event-free survival) (Fig. 28-1). Importantly, patients with normal MPI had a 5-year death/MI free rate of 92%, yielding an annual event rate of 1.6% per year. An interesting finding in this study was that ischemia proximal to bypass anastomosis was not associated with adverse outcome.
In a series of 75 patients undergoing stress MPI a mean of 38 months after CABG, the best predictor of cardiac events was the summed reversibility score (SRS), reflecting the extent of jeopardized viable myocardium.13 This variable added significant incremental prognostic value to clinical and exercise data and nearly doubled the chi-square of the predictive model. A meta-analysis of functional testing for graft stenosis or progression of native disease showed superior sensitivity for stress MPI compared to exercise treadmill testing (ETT) (68% versus 45%), with similar specificity (84% versus 82%, respectively).14
Assessment Late After CABG (Beyond 5 Years)
Several studies have consistently shown that stress MPI performed late after CABG predicts future cardiac death and MI.15–20 Palmas and colleagues evaluated 294 patients undergoing stress 201Tl MPI at least 5 years after CABG.16 They found that the SRS reflecting jeopardized viable myocardium was significantly related to future death or MI and had significant incremental predictive value to clinical and exercise data, doubling the predictive model chi-square. Stress nuclear MPI data in patients 5 years after CABG have also been shown to add significant prognostic value to angiographic data, doubling the predictive model chi-square for cardiac death/MI when added to clinical, exercise, and angiographic data.17 Significant multivariate predictors of cardiac death or MI included the extent of the perfusion defect, multivessel perfusion defects, and increased 201Tl lung uptake. Zellweger and colleagues also found that the extent of reversible defects in patients undergoing stress MPI more than 5 years after CABG was a significant multivariate predictor of cardiac death.18
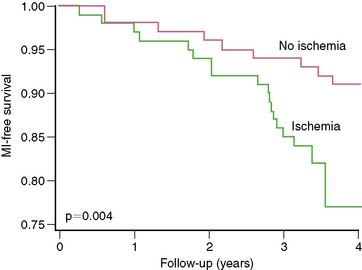
Even in asymptomatic patients presenting 6 to 7 years out from their CABG, stress nuclear MPI is a powerful predictor of death or MI.19 Reversible perfusion defects and exercise capacity were multivariate predictors of death or MI when clinical factors were adjusted. The presence of any reversible defect was associated with a 13% death/MI rate over 3 years, compared to 7% without reversible defect (P = 0.004) (Fig. 28-2), with separation in risk appearing to grow over additional time.
EVALUATION AFTER PERCUTANEOUS CORONARY INTERVENTION
Early After PCI
There is little role for performing screening MPI before 6 weeks, because generally the restenosis phenomenon does not begin to occur before this time. Nevertheless, in patients presenting with chest pain, stress MPI may be helpful in ruling out ischemia as a basis even in this time frame, although interpretation of images requires an understanding of the impact of coronary intervention on coronary responsiveness to hyperemic stimuli. In the days of balloon angioplasty without stent placement, the initial vascular response to reduction of diameter narrowing with mechanical plaque rupture was variable. Several studies showed that perfusion defects on stress MPI early after angioplasty predicted development of restenosis.20–23 Furthermore, even in arteries that do not show restenosis early, perfusion defect on stress MPI can be seen at a mean 9 days after angioplasty and progressively resolves on serial imaging at 3 and 7 months after angioplasty.24 Similar findings have been described for coronary stenting, despite generally more satisfactory initial angiographic results.25–28 The initial perfusion defect may reflect residual vessel obstruction, regional microvascular distal dysfunction in the territory of the dilated artery, angiographically unapparent dissections, or new accumulations of focally extruded plaque. The initial perfusion defect and diminished coronary flow reserve may also reflect endothelial dysfunction and medial injury without coronary stenosis that resolves with time.29–31 These factors contribute to decreased specificity of MPI early after PCI, which substantially impairs the clinical utility of stress MPI in this time frame.
3 to 6 Months After PCI
This time frame represents the general period during which the restenosis phenomenon becomes completed. A large body of literature has evaluated the role of stress nuclear MPI in this clinical setting. It supports the use of stress MPI for detecting the presence of silent or symptomatic restenosis and documents its incremental prognostic value compared to treadmill testing and angiography, its cost-effectiveness, and its overall ability to predict cardiac events and guide management decisions.32–41
In a series of 116 patients 6 months out from angioplasty, stress nuclear MPI and treadmill testing were compared for predicting restenosis.42 SPECT MPI had superior sensitivity (93% versus 52%), specificity (77% versus 64%), and accuracy (86% versus 57%) compared to treadmill testing. In addition, MPI was able to correctly identify the culprit vessel with 86% accuracy. Importantly, many patients with angiographic restenosis are asymptomatic, and the accuracy of stress MPI is not affected by manifestation of the symptoms. Hecht and colleagues reported that in their series of 116 patients, angiographic restenosis occurred in 61% of symptomatic and 59% of asymptomatic patients.43 The ability of stress MPI to detect restenosis was similar: 96% and 91% sensitivity for asymptomatic and symptomatic patients, respectively, with 75% and 77% specificity, respectively. The sensitivity of stress EKG was substantially lower, 40% and 59%, respectively for silent and symptomatic patients. A series by Marie et al. confirmed that asymptomatic restenosis is common but well detected by stress MPI. Silent and symptomatic restenosis occurred at equal rates, and stress nuclear MPI detected 100% of the lesions, whereas stress ECG detected only 25%.44 Numerous other studies, including a meta-analysis of publications between 1975 and 2000, have showed sensitivity of 80% to 90% for detecting restenosis, compared to 40% to 50% for treadmill exercise testing.45–47
Late After PCI
During the late time period after PCI, progression of disease in untreated vessel segments is more prevalent than restenosis.48–52 Beyond simply detecting the presence or absence of restenosis after PCI, stress nuclear MPI performed outside the window of restenosis has the ability to predict major cardiac events, including death or MI. In a series of 211 patients undergoing stress 201Tl MPI 1 to 3 years after balloon angioplasties, future cardiac death or MI was best predicted by the summed stress score, which will reflect the extent of ischemia plus scar.53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree