Overview
Nuclear cardiology is an integral part of cardiovascular practice, with stress nuclear cardiology procedures accounting for more than one third of all stress tests performed by cardiologists and, until recently, sustaining growth rates approaching 20% per year. This chapter provides a synopsis of nuclear cardiology procedures and the published evidence of their role in the diagnosis and risk assessment of patients with suspected or known coronary artery disease (CAD). In this chapter, CAD refers to the presence of an obstructive stenosis, in contradistinction to coronary atherosclerosis, which denotes the presence of any atherosclerotic plaque in the coronary arteries.
The Anger scintillation camera, the imaging device used today for the vast majority of all nuclear cardiology procedures except positron emission tomography (PET), became clinically available in the late 1960s. By providing dynamic images of the cardiac distribution of radioactivity, this camera marked the beginning of clinical nuclear cardiology. The commercial availability in 1976 of thallium-201 (201Tl) initiated the broad application of clinical myocardial perfusion scintigraphy. Two-dimensional planar 201Tl imaging was quickly shown to be useful for detection of CAD and, in the early 1980s, was demonstrated to be highly valuable in risk stratification of the CAD patient. Also in the early 1980s, single-photon emission computed tomography (SPECT), using a rotating Anger camera detector, became widely available, increasing the ability to localize and quantify regional myocardial perfusion defects. In 1990, technetium-99m (99mTc)-sestamibi was approved for use in the United States, followed shortly thereafter by another 99mTc-based agent, 99mTc-tetrofosmin. The higher myocardial count rates of the 99mTc agents made it possible to perform electrocardiogram (ECG)-gated SPECT myocardial perfusion imaging (MPI) by obtaining images from the different parts of the cardiac cycle (gated SPECT). By the late 1990s, the widespread availability of dual-detector cameras and dramatic increases in speed of computer systems allowed gated SPECT MPI to become a common clinical routine.1 By 2003, >90% of SPECT MPI used gated SPECT, providing routine objective clinical assessments of rest and stress myocardial perfusion and function. Radionuclide angiography played a prominent role in nuclear cardiology in decades past, but by the 1990s, the use of this modality was largely replaced by echocardiography. Gated SPECT MPI now makes up >95% of all nuclear cardiology procedures and thus is the primary focus of this chapter.
The American College of Cardiology (ACC)/American Heart Association (AHA)/American Society of Nuclear Cardiology (ASNC) Guidelines for the Clinical Use of Cardiac Radionuclide Imaging provide a useful, comprehensive review of the recommendations for the use of nuclear cardiology techniques.2 Importantly, in 2009, the Appropriate Use Criteria of the ACC and ASNC were revised, providing a summary of scenarios in which the use of SPECT or PET MPI is evaluated as indicated, uncertain, or not indicated.3
SPECT Myocardial Perfusion Imaging
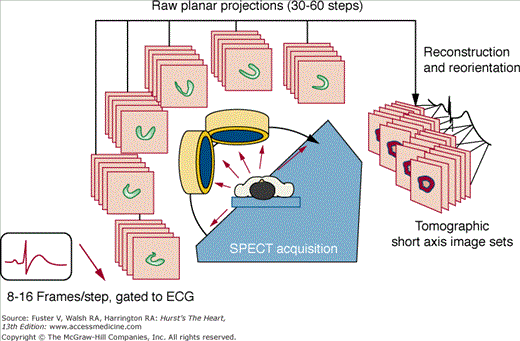
SPECT MPI is performed using a scintillation camera and an intravenously injected radiopharmaceutical that distributes to the heart in proportion to regional myocardial perfusion. Various standardized SPECT MPI protocols are used for imaging at rest and after exercise or pharmacologic stress. For SPECT, conventional scintillation camera detectors rotate to cover a 180-degree arc around the patient in a semicircular or elliptical fashion, collecting a series of planar projection images at regular angular intervals. The three-dimensional (3D) distribution of radioactivity in the myocardium is then mathematically reconstructed from the two-dimensional projections, and the resulting data are displayed in series of slices in the short axis, vertical long axis, and horizontal long axis orientations. For gated SPECT (Fig. 21–1), the feature that distinguishes it from nongated SPECT is that the projection images are acquired in 8 to 16 phases of the cardiac cycle based on ECG triggering (gating). Reconstruction of a summation of the frames is used to assess myocardial perfusion, and reconstruction of each of the separate phases is used to evaluate ventricular function. Recently, solid-state detector cameras with new acquisition geometries have been introduced that do not require detector rotation around the patient and offer the potential for reducing the time and the patient radiation dose associated with SPECT MPI.3a,3b
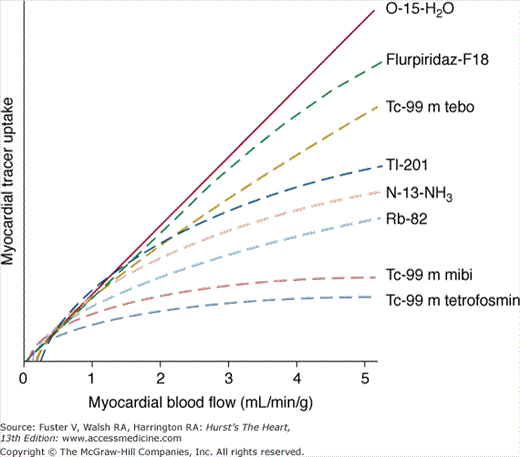
The radiopharmaceuticals used for gated SPECT MPI share the characteristic that they are accumulated in viable myocardium in proportion to regional myocardial blood flow (for a review of these agents, see Taillefer4). The concept that CAD can be detected with this approach is based on the ability to detect a reduction in myocardial perfusion in a region supplied by a significantly stenosed vessel compared with a normal region during hyperemia. The relationship between the degree of coronary artery narrowing and the maximal hyperemic response was first elucidated by Gould in 1974.5 Resting myocardial perfusion is normal until the luminal diameter narrowing of a coronary artery exceeds 90% to 95%. With maximal coronary hyperemia produced by dipyridamole, Gould demonstrated a progressive decrease in the hyperemic response associated with increasing degrees of stenosis after the threshold of 50% diameter narrowing had been reached. Since the work of Gould, it has become widely considered that a significant reduction in maximal hyperemia does not generally occur until stenosis severity exceeds 70%.6 These considerations imply that all forms of stress testing will be insensitive for the detection of coronary atherosclerosis until a hemodynamically significant lesion has developed. Hemodynamically significant lesion could be fixed or dynamic, such that spasm or paradoxical vasoconstriction during stress could result in a reduction of peak hyperemic perfusion even in the absence of a fixed >50% to 70% stenosis by angiography.7 The ability of SPECT MPI to assess the hyperemic response is related to the degree of extraction of the tracer during its first pass through the myocardium. The amount of the injected tracer in the myocardium at the time of imaging (net extraction) is a function of the proportion extracted by the myocardium (extraction fraction) and the amount retained at the time of imaging. With the exception of oxygen-15 water, which is generally used only for research purposes, all available SPECT or PET radiopharmaceuticals (see Chap. 25) manifest a roll-off in this extraction as coronary flow increases to high levels. Because mild stenoses may produce a decrease in flow only at very high flow rates, the net extraction is one of the most important characteristics of perfusion tracers. Figure 21–2 illustrates the net extraction of the various SPECT and PET myocardial perfusion tracers. Figure 21–3 illustrates a new PET tracer (Flurpiridaz-F18) currently in clinical trials and compares it to 99mTc-sestamibi.8
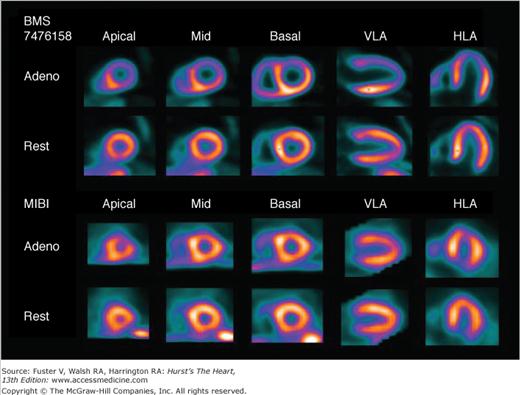
Figure 21–3.
Case example of adenosine (Adeno) stress myocardial perfusion imaging study from the Lantheus Medical Imaging phase II clinical trial to evaluate Flurpiridaz-F18 as a myocardial perfusion imaging agent. Subject is an 87-year-old woman with shortness of breath and prior percutaneous coronary intervention. Positron emission tomography (PET) perfusion images using Flurpiridaz-F18 (top two rows) show a large severe reversible defect in the left anterior descending (LAD) coronary artery territory, in contrast to the technetium-99m–sestamibi (MIBI) single-photon emission computed tomography (SPECT) images (bottom two rows) obtained after 3 days in which the reversible defect is much smaller. Invasive coronary angiography revealed a 100% occlusion due to in-stent restenosis in the LAD. HLA, horizontal long axis; VLA, vertical long axis.
The monovalent cation thallium is a potassium analog that is avidly extracted from the blood by myocardial cells. A cyclotron-generated radionuclide with a half-life of 73 hours, 201Tl emits photons at 68 to 80 keV (90% abundance) and at 167 keV (10% abundance). Because of its relatively long half-life of 73 hours, the absorbed radiation dose per unit injected is higher with 201Tl than with 99mTc agents. For the same radiation dose to the patient, approximately one-tenth as much radioactivity with 201Tl can be injected compared with 99mTc, making imaging times longer and generally providing lower information density (“counts”) in the clinical images. Despite its less favorable radiation dosimetry, 201Tl has superior physiologic properties for MPI compared with the 99mTc agents. Unlike the 99mTc agents, with 201Tl, the linear relationship between blood flow and 201Tl uptake is maintained during hyperemic stress up to high levels of flow (approximately >3 mL/min/g) before the previously noted roll-off in uptake occurs4 (see Fig. 21–2). As an unbound potassium analog, 201Tl redistributes over time9,10; its initial distribution is proportional to regional myocardial perfusion, and at equilibrium, the distribution of 201Tl is proportional to the regional potassium pool, which represents viable myocardium.11 By virtue of these properties, myocardial perfusion defect reversibility can be imaged at two separate times with a single injection of 201Tl. The mechanisms of 201Tl redistribution are differential washout rates between hypoperfused but viable myocardium and normal zones, as well as wash-in from the blood to initially hypoperfused zones. For practical purposes, the time it takes for 201Tl to reach its ultimate distribution in viable myocardium varies, such that delayed redistribution imaging (at 24 hours rather than 3-4 hours) is commonly used to extract the greatest amount of information from thallium studies regarding defect reversibility and myocardial viability.12
The washout rate of 201Tl is a function of the concentration gradient between the myocardial cell and the blood. Hyperinsulinemic states drive thallium into cells, leading to reduced blood 201Tl concentrations and slow redistribution; thus, carbohydrates are avoided prior to and for 4 hours following 201Tl injection.4 An inverse relationship between the degree of coronary stenosis and subsequent redistribution of 201Tl (ie, late redistribution) has been reported.4,13
Technetium-99m is produced from a molybdenum-99 generator, has a half-life of 6 hours, and emits monoenergetic gamma rays at 140 keV. As noted earlier, for the same radiation dose to the patient, approximately 10 times as much radioactivity as 201-Tl can be injected with 99mTc improving image quality. Following extraction from the blood, 99mTc-sestamibi and 99mTc-tetrofosmin are quickly bound by mitochondria, and only a limited amount of myocardial washout (or wash-in) occurs over time.4,14 Thus, with these tracers, separate rest and stress injections are required to evaluate perfusion defect reversibility. A drawback of both perfusion tracers is that they exhibit a greater roll-off of extraction with increasing coronary flow and lower net extraction by the myocardium than thallium.15 Another drawback of the 99mTc tracers is their gastrointestinal excretion, resulting in greater hepatic and gastrointestinal uptake than thallium, not infrequently interfering with inferior myocardial wall visualization, particularly after rest injection.4
From a practical standpoint, a main advantage of the 99mTc agents is greater flexibility than provided by 201Tl. Because of their mitochondrial binding, they do not require that imaging commence soon after the stress injection for maximal sensitivity in detecting perfusion defects. In contrast, with 201Tl, imaging must be performed soon after stress testing (recommended to begin within 10-15 minutes) because rapid redistribution can cause mild perfusion defects to become undetectable by the time imaging begins. Thus, with 201Tl, if soft tissue attenuation or patient motion compromises a study, the benefit of repeating the acquisition is questionable. With 99mTc sestamibi or tetrofosmin, however, current guidelines allow that imaging can commence as late as 2 hours after injection. This allows a laboratory to run stress tests on several patients in short succession and then to perform the imaging procedures over the ensuing 2 hours. Additionally, when patient motion, soft tissue attenuation, or other artifact is considered to be responsible for the production of a perfusion defect, image acquisition can simply be repeated (eg, in the prone position16) with little concern that defects may no longer be detectable due to redistribution of the tracer.
In May 2009, Atomic Energy of Canada Ltd announced that the nuclear reactor at Chalk River, Ontario, Canada, would be out of service due to a heavy water leak and would remain out of service until at least early 2010. This has led to a worldwide shortage of molybdenum-99m, which is used to manufacture 99mTc generators; thus, there have frequently been days when the nuclear cardiology community has less 99mTc-sestamibi or 99mTc-tetrofosmin than needed. Alternatives to rest/stress 99mTc-sestamibi or 99mTc-tetrofosmin imaging include pharmacologic rubidium-82 PET imaging, dual-isotope (rest 201 Tl/stress 99mTc-sestamibi or 99mTc-tetrofosmin) imaging, or stress/redistribution 201 Tl imaging. There is sufficient supply of cyclotron-produced 201Tl to supply the world’s need of radiopharmaceutical for SPECT MPI, without needing nuclear reactors.
Exercise stress is preferred over pharmacologic stress for use with SPECT MPI in patients who can exercise adequately because it allows assessment of exercise capacity, heart rate (HR) and blood pressure (BP) responses, and symptoms, as well as ST-segment response, providing additional clinical information that can be useful in clinical decision making.2 For exercise SPECT MPI, an indwelling intravenous line is inserted before exercise, the tracer is injected at maximal stress, and exercise is continued for an additional 1 minute at peak workload. Although it is desirable for treadmill exercise to be symptom limited, achieving 85% of the estimated maximum predicted HR (220 –age) has been the traditional cutoff for an acceptable level of stress.2 It has been shown that if the perfusion tracer is injected at <80% maximal predicted HR (MPHR), a normal SPECT MPI scan does not confer as low a risk as that associated with greater workloads.17 Postexercise stress imaging routinely commences 15 minutes after stress, but with the 99mTc perfusion tracers, because of the absence of significant redistribution, imaging can begin up to 2 hours after stress injection. The advantage of starting imaging earlier is that it increases the opportunity to observe stress-induced wall motion abnormalities (“stunning”) during gated SPECT acquisition.18 However, initiating image acquisition too early, before the patient has not fully recovered from stress, potentially introduces artifactual defects (upward creep of the heart)19 related to increased depth of respiration very early after stress, causing the heart to gradually move cephalad during the early portion of SPECT acquisition.
For patients who cannot achieve an adequate level of exercise (≥85% of MPHR2), pharmacologic stress testing is the preferred approach to stress.20 The preferred pharmacologic stress agents for SPECT MPI are coronary vasodilators: adenosine, regadenoson, or dipyridamole. These agents provide a three- to five-fold increase in coronary flow. Dipyridamole blocks the cellular reuptake of adenosine, increasing the extracellular adenosine concentration. Increased extracellular adenosine results in coronary vasodilation. Regadenoson is a recently approved selective A2A adenosine receptor agonist, recently approved for clinical use in the United States.21 Methylxanthines, such as theophylline or caffeine, block adenosine binding and can reduce the coronary vasodilation effects of adenosine, regadenoson, or dipyridamole, leading to false-negative stress perfusion studies. In general, discontinuation of caffeine-containing medicines, foods, or beverages for 24 hours prior to adenosine or dipyridamole and 12 hours prior to regadenoson is recommended.2,22 A recent small study by Zoghbi and colleagues suggested that one cup of coffee does not mask the presence or severity of reversible defects induced by adenosine SPECT imaging.22a Whether caffeine masks perfusion defects with regadenoson is currently under study. To avoid overstimulation of adenosine receptors, use of dipyridamole-containing medications in the 48 hours prior to adenosine or regadenoson administration should be avoided.22 The diagnostic accuracies of SPECT MPI using exercise or vasodilator stress have been found to be equivalent, despite the higher coronary flow rates associated with the vasodilators.23
Vasodilator stress is frequently associated with chest discomfort and shortness of breath, even in normal subjects, and thus, unlike exercise, with vasodilator stress, these symptoms are not considered to indicate the presence of ischemia. Vasodilator stress frequently causes an increase in HR and a decrease in BP. With adenosine and dipyridamole, these changes are mild, but with regadenoson, the HR increase has been reported to be more marked.21 The clinical assessment and hemodynamic responses to vasodilators are not useful in identifying patients in whom the pharmacologic effects of adenosine or dipyridamole may have been blocked by caffeine; failure of HR or BP to change with adenosine stress does not imply lack of myocardial perfusion response.24 However, recent data have suggested that resting tachycardia and failure to mount a reflex tachycardia in response to vasodilator stress are associated with an increase in mortality risk.25
Dipyridamole is usually infused at 0.56 mg/kg/min for 4 minutes, but some investigators have recommended increasing the dose by 50%.23 The maximal effect occurs approximately 3 to 4 minutes after end of the infusion, and hyperemia persists for approximately 20 to 30 minutes. Mild transient adverse effects are common, including chest pain, shortness of breath, dizziness, and flushing. Severe adverse effects are rare, being noted in only 1 of 10,000 patients.26 Adverse effects can usually be reversed by intravenous aminophylline, usually 75 to 125 mg. Because of the potential adverse effect of severe bronchospasm, dipyridamole is contraindicated for asthmatics.
Adenosine is infused intravenously (140 μg/kg/min), usually over 4 to 6 minutes, with radiopharmaceutical administration at 2 to 3 minutes of infusion.23,27 Transient adverse effects occur more frequently than with dipyridamole,23 but because of the brief duration of action (~13 seconds), reversal with aminophylline is rarely used. With adenosine, there is an increased incidence of transient advanced atrioventricular (AV) block. Adenosine is considered contraindicated for patients with greater than first-degree AV block, sick sinus syndrome, or bronchospasm.
Selective A2A agonists have the potential for reducing the high frequency of uncomfortable systemic adverse effects and the risk of bronchoconstriction in asthmatics that are associated with adenosine and dipyridamole (Fig. 21–4). At the time of this writing, regadenoson is the only selective A2A adenosine agonist to receive US Food and Drug Administration (FDA) approval, based on the results of two phase III studies that demonstrated equivalency to adenosine for the detection of reversible perfusion defects.21 Unlike adenosine and dipyridamole, which require infusions and weight-based dosing, regadenoson is directly injected over a 10-second period with a fixed dose (0.4 mg) in a prefilled syringe, without requiring an infusion pump.22 Adverse effects have been reported to be better tolerated than adenosine.21 The radiopharmaceutical is injected 10 to 20 seconds after intravenous injection of regadenoson.22 Regadenoson is considered contraindicated for patients with greater than first-degree AV block or sick sinus syndrome.22 Two small studies have reported the use of regadenoson in patients with chronic obstructive pulmonary disease or asthma, but the data indicate that the use of regadenoson in patients with these conditions must be performed with appropriate precautionary measures in case bronchospasm should occur.22
Figure 21–4.
Time course of changes in coronary conductance caused by regadenoson (yellow), binodenoson (green), CGS-21680 (red), and adenosine (blue). Reproduced with permission from Gao et al.234
It has become increasingly common to combine vasodilator stress with low-level exercise.28 With dipyridamole, low-level exercise commences after the end of dipyridamole infusion, and with adenosine, exercise is performed during the infusion. With regadenoson, exercise can commence before injection, allowing the vasodilator and tracer to be injected at the point of maximal exercise. Exercise even at low levels reduces splanchnic blood flow and, thereby, hepatic uptake of 99mTc-sestamibi or 99mTc- tetrofosmin, facilitating early postinfusion imaging with these tracers (as early as 10-15 minutes following injection) compared to the 1-hour delay required when adjunctive exercise is not performed. Another key benefit is a marked reduction in the frequency and severity of adverse effects from the vasodilator stress agents, possibly caused by the increased attention to the task of walking in patients who usually have reduced exercise capacity. Being able to perform low-level exercise also provides additional prognostic information.29 Given all of these advantages, it is widely recommended that low-level exercise be combined with vasodilator stress for MPI when possible, with the exception of patients with left bundle-branch block (LBBB) or paced rhythm in whom it is preferable not to have the increased HR associated with exercise.
With exercise stress, patients with LBBB frequently demonstrate reversible defects in the septal wall in the absence of CAD. Because of the relationship between the increase in HR and the presence of perfusion defects without CAD in LBBB and paced ventricular rhythms, vasodilator stress is preferred over exercise in these patients, and the vasodilator stress protocols are performed without adjunctive low-level exercise.2 Stress modalities that do not increase HR as markedly as exercise, such as adenosine or dipyridamole stress without walking, are generally considered preferable in LBBB patients.30 With exercise SPECT MPI, the perfusion defect associated with LBBB in the absence of left anterior descending CAD most commonly involves the interventricular septum with sparing of the apex of the left ventricle, a pattern that would be uncommon for left anterior descending CAD.31 Myocardial perfusion defects in the inferior and apical walls have also been reported in the absence of CAD in patients with prolonged right ventricular (RV) pacing.23
Unlike exercise stress testing, which is generally avoided in patients with significant aortic stenosis, vasodilator stress is considered safe and effective in patients with moderate aortic stenosis.32 However, in patients with severe symptomatic aortic stenosis, any form of stress testing (exercise, vasodilator, or dobutamine) is considered contraindicated.22
An alternative to vasodilator stress is inotropic stress with dobutamine. At present, dobutamine stress is usually reserved for patients with asthma, patients with chronic obstructive pulmonary disease with bronchospasm, or patients who have ingested caffeine close to the time of testing. Dobutamine stress results in a lower-rate pressure product than exercise and a lower peak coronary blood flow with vasodilator stress. Adverse effects, including ventricular irritability, are more common than with the vasodilator stress. The protocol used is the same as that used for dobutamine stress echocardiography. As with echocardiography, atropine is commonly used in addition >85% if the maximal predicted heart rate is not achieved.
In general, for purposes of diagnosis or initial risk stratification, stress nuclear testing is performed with the patient off anti-ischemic medications,2 because these medications may limit the development of ischemia during the stress test. When feasible, use of β-blockers or long-acting calcium channel blockers should be discontinued for 48 hours before stress imaging, and long-acting nitrates should be discontinued at least 12 hours before stress imaging.2 Although initial risk stratification studies are generally performed off cardiac-active medications, potentially useful clinical information can be derived from exercise or pharmacologic SPECT MPI performed with the patient on cardiac medications.2
With respect to pharmacologic stress testing, it has been reported33 that compared with SPECT MPI performed off medications, dipyridamole SPECT MPI performed on medications was associated with smaller and less reversible perfusion defects and approximately a one-third lowering of overall vessel sensitivity (62% vs 92%).31 A recent study has demonstrated that acute β-blockade reduces the size of adenosine stress myocardial perfusion defects and doubles the likelihood of false-negative studies (14.3% vs 28.6%).34 It has been shown that patients with normal exercise SPECT MPI who achieve <80% to 85% MPHR are at greater risk than those who achieve maximal workload.17,35 Thus, recommendations are that patients be off of caffeine prior to exercise or vasodilator stress SPECT MPI.2 If a patient fails to achieve 85% of MPHR during exercise, regadenoson can be injected prior to tracer injection while the patient is still exercising, or stress testing with any of the vasodilators can be immediately substituted for exercise prior to stress tracer injection.
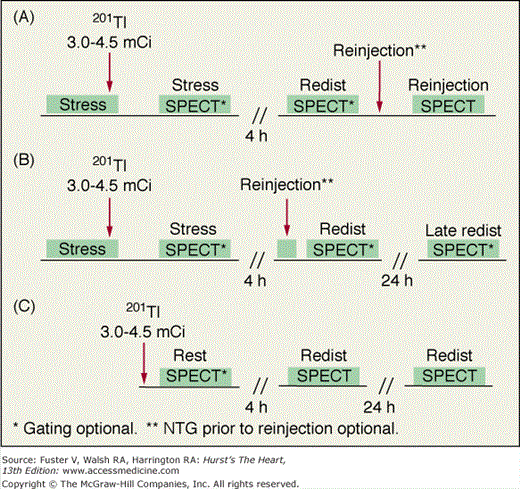
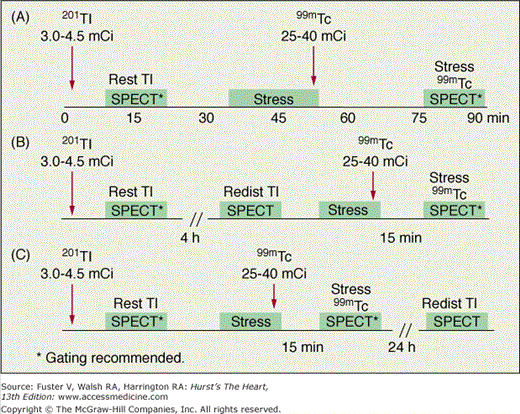
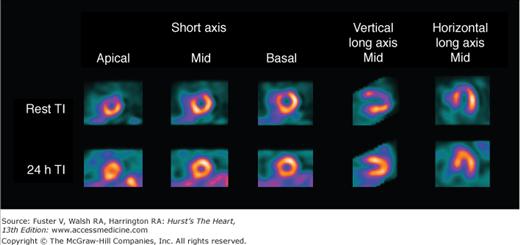
With 201Tl, a variety of SPECT protocols are available (Fig. 21–5). When 201Tl is the only radiopharmaceutical, the usual protocol uses some combination of stress with redistribution and/or reinjection SPECT MPI (see Fig. 21–5A). The initially described protocol using simply stress and redistribution imaging is still commonly used. It was later reported that reinjection with a small dose of 201Tl could improve detection of perfusion defect reversibility. Two or three acquisition sequences are performed on the day of stress testing. If nonreversible (fixed) defects are found at 4-hour imaging (see Fig. 21–5B), additional 24-hour imaging has been reported to improved detection of defect reversibility.12,13 Sublingual nitroglycerin prior to 201Tl reinjection may reduce the need for 24-hour imaging. Rest/redistribution 201Tl SPECT MPI is commonly used for resting ischemia/viability testing9 (see Fig. 21–5C). Even with rest 201Tl SPECT, 24-hour imaging can result in additional redistribution compared to 4-hour imaging.12,23 With 201Tl SPECT, the timing of initial poststress acquisition is important because early 201Tl redistribution can decrease sensitivity for CAD if poststress imaging is delayed.
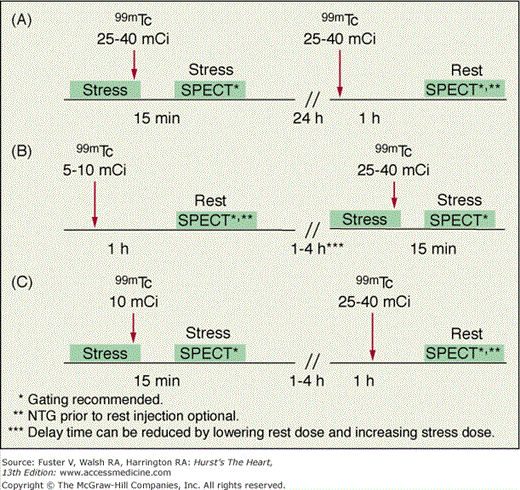
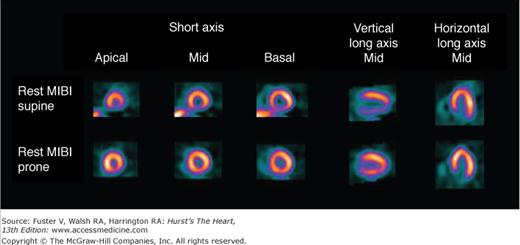
Because uptake and radiation dosimetry of these compounds are similar, the recommended acquisition protocols are the same. The radiation dosimetry of these agents is better than that of 201Tl. Because of the absence of clinically significant redistribution, separate rest and stress injections are needed with 99mTc-sestamibi or 99mTc-tetrofosmin SPECT (Fig. 21–6)23 to assess reversibility of perfusion defects. With these agents, if imaging artifact is suspected on supine images, additional prone imaging can be performed to increase the specificity of SPECT MPI (Fig. 21–7).16,23,36
Figure 21–7.
Rest sestamibi (MIBI) single-photon emission computed tomography (SPECT) images in the supine position (top) and prone position (bottom) in a 55-year-old patient with a low likelihood of coronary artery disease. Prone images are normal, demonstrating that the apparent inferior wall perfusion defect on the supine images is secondary to soft tissue attenuation. Normal wall motion was noted on gated SPECT myocardial perfusion imaging (MPI). Gated resting MPI: end-diastolic volume = 55 mL, end-systolic volume = 35 mL, and ejection fraction = 55%. Reproduced with permission from Berman and Germano.36
A variety of protocols can be used with these agents, including 2-day stress/rest, same-day rest/stress, same-day stress/rest, and dual-isotope. From the standpoint of defect contrast and image quality, the 2-day stress/rest protocol is ideal (see Fig. 21–6A). Both stress and rest studies are obtained after injection of high doses of 99mTc-sestamibi or 99mTc-tetrofosmin, allowing acquisition of consistent high-count images with the drawback being the need for the patient to return for an additional image the following day. The most common 99mTc agent protocol is same-day low-dose rest/high-dose stress (see Fig. 21–6B).23 Although convenient, it has the disadvantage of reduction in stress-defect contrast, as approximately 15% of the radioactivity observed at the time of stress imaging comes from the pre-existing resting injection. The same-day low-dose stress/high-dose rest sequence (see Fig. 21–6C) has the advantage of image acquisition times that are the same as those used for stress 201Tl imaging, facilitating mixing of these two protocols. The principal drawback of this approach is that the count rates associated with the stress image set are low. With either the 1- or 2-day stress/rest sequences, an advantage is the ability to perform the stress image only.23 Viability assessment with 99mTc-sestamibi or 99mTc-tetrofosmin may be improved by the administration of nitroglycerin prior to the rest-injection study.23,37
An alternative to these 99mTc-sestamibi or 99mTc-tetrofosmin protocols is a rest 201Tl/stress 99mTc-sestamibi or 99mTc-tetrofosmin dual-isotope SPECT (Fig. 21–8),27 taking advantage of the Anger camera’s ability to collect data in different energy windows. It is usually performed with separate rest/stress acquisitions, including redistribution thallium images either before the stress study (see Fig. 21–8B) or at 24 hours (after stress) (see Fig. 21–8C) if a rest defect is present. Advantages of this approach are increased efficiency and the ability to assess resting perfusion defect reversibility (detecting resting ischemia by 201Tl redistribution images). The separate acquisition approach does not require correction for cross-contamination between the two radioisotopes,27 which is important for simultaneous dual-isotope approach, a potentially efficient approach not yet in general clinical use.
Figure 21–8.
Variations of the resting thallium-201 (201Tl)/technetium-99m (99mTc)-sestamibi or 99mTc-tetrofosmin dual-isotope single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) protocol. Most commonly, only rest and stress imaging is performed (A). When resting defects are present, redistribution imaging is recommended and may be performed before (B) or 24 hours after (C) injection of the technetium perfusion agent.
In any protocol using the rest/stress sequence, the resting images can be inspected prior to stress testing (Fig. 21–9), enabling the identification of patients with unexpected rest perfusion defects, usually secondary to critical CAD, in whom unnecessary stress testing can be avoided.38
Figure 21–9.
Rest and 24-hour redistribution thallium-201 (201Tl) single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) of a 75-year-old male with atypical angina showing a large amount of resting ischemia in the left anterior descending territory, which subsequently revealed a 95% proximal stenosis by coronary angiography. Of note, the left ventricle was larger at rest than at the time of redistribution imaging. The stress SPECT MPI study was cancelled in this patient because of the unexpected perfusion defect.
Recently, the dual-isotope approach has been decreasing in usage due to the less favorable radiation dosimetry of thallium compared with 99mTc-sestamibi or 99mTc-tetrofosmin, but it remains a viable option given the worldwide shortage of 99mTc generators. An alternative rapid “reverse dual” protocol (stress thallium/rest 99mTc-sestamibi or 99mTc-tetrofosmin) using lower radionuclide doses and camera systems capable of faster acquisitions has recently been reported, allowing the use of thallium-201 as the stress agent (preferable due to superior net extraction compared with 99mTc-sestamibi or 99mTc-tetrofosmin) while maintaining a relatively low radiation burden for the patient.39
One of the most difficult problems in interpretation of SPECT MPI is the differentiation of artifactual from true perfusion defects. Imaging the patient in the prone position with the nuclear detector rotating underneath the patient decreases the frequency of attenuation artifacts in the inferior wall and also decreases motion artifact (see Fig. 21–7).40 However, prone imaging may create artifactual anteroseptal defects caused by the more pronounced sternal attenuation in this position.23 As a consequence, combined prone and supine imaging with the sestamibi or tetrofosmin has been described. Hayes and coworkers40 reported that patients with inferior wall defects on supine SPECT MPI but normal prone SPECT MPI have as low a risk of subsequent cardiac events as patients with normal supine-only studies. A quantitative method for computer-based interpretation of combined supine and prone sestamibi SPECT images has recently been reported.16 With this approach, supine and prone images are compared with their respective normal databases, and only regions found concordantly abnormal by both positions are considered abnormal. The combined quantitation has demonstrated an increase in the normalcy rate without a loss of sensitivity.
Several camera manufacturers have recently provided hardware and software implementation of attenuation correction protocols. In general, these corrections are imperfect, reducing but not eliminating apparent perfusion defects caused by soft tissue attenuation in normal patients.41 Several reports have compared the diagnostic accuracy of attenuation correction and non–attenuation correction SPECT using a variety of commercially available approaches. In general, these have demonstrated improved specificity with no change in overall sensitivity.42,43 Despite these considerations, attenuation correction is not yet widely used, possibly because of the lack of a common approach to its commercial implementation by camera manufacturers.41
Standard myocardial perfusion SPECT imaging protocols consist of a rest acquisition followed by at least one poststress acquisition after exercise or pharmacologic stress. Stress only imaging has been proposed to have several potential benefits.44-47 Because the rest study is not performed, there is reduced imaging time, lower radiation exposure, and lower cost.45 Limitations of the stress only protocol are that transient ischemic dilation and stunning (decrease in ejection fraction or wall motion on poststress compared with rest imaging or development of poststress worsening of wall motion) cannot be assessed.45 The approach can also result in a higher false-positive rate than rest/stress imaging because failure of a minor defect to change between rest and stress can be used to identify artifact. Adding attenuation correction or prone imaging may increase the percentage of normal stress images and thus reduce the percentage of patients who have to return for rest imaging.45 Because patients undergoing pharmacologic stress are, as a group, at higher risk, it has been suggested that the stress only approach may be more appropriate for patients who can exercise.45 Prognosis has been assessed in a study by Gibson et al.46 They demonstrated, in 729 patients with chest pain and a low to intermediate pretest probability of CAD who had normal stress perfusion (attenuation correction needed in 37%), that the incidence of cardiac events at 22 months was 0.6%.46 Similar findings in a larger group have been recently reported by Chang et al.46a
Basics of Interpretation
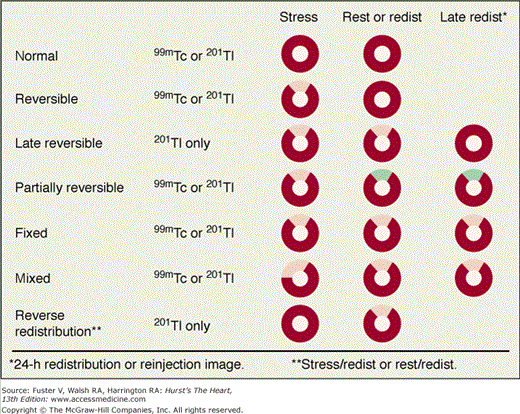
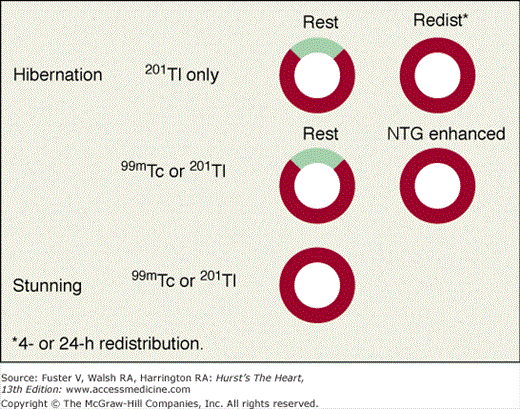
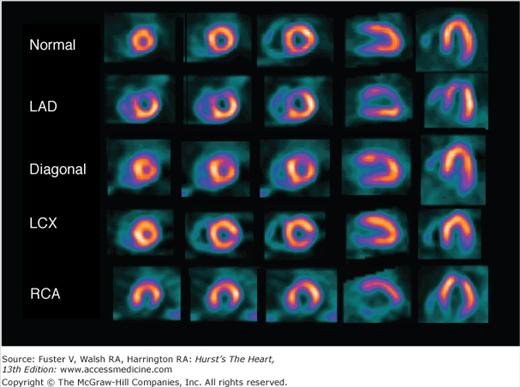
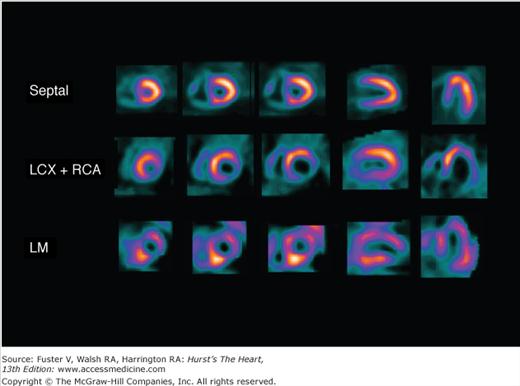
Perfusion defects are characterized by their type as well as their extent and severity. The various defect types are illustrated in Fig. 21–10 for stress and rest SPECT MPI and in Fig. 21–11 for rest SPECT MPI viability patterns. The distribution of SPECT abnormalities provides information regarding the location of coronary artery stenoses. Representative examples of SPECT defect locations associated with individual coronary artery stenoses are illustrated in Figs. 21–12 and Fig. 21–13.
Figure 21–10.
Patterns of stress/rest or redistribution (redist) single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) defects. Red represents normal tracer update; pink represents a definite perfusion defect; green represents less severe but still definite perfusion defect (seen in the partially reversible defect example). 99mTc, technetium-99m–sestamibi or –tetrofosmin; 201Tl, thallium-201.
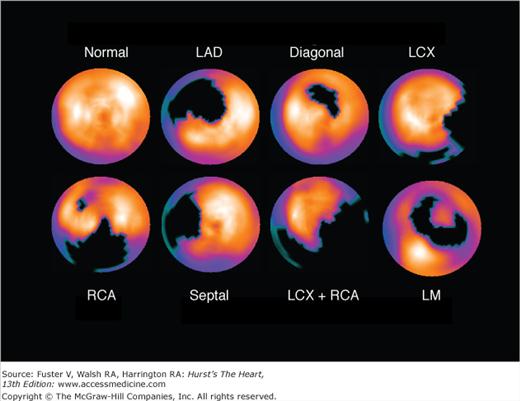
Figure 21–12.
Examples of typical stress perfusion patterns corresponding to normal (top) and various single-territory abnormalities. Coronary angiographic findings in these patients were as follows: left anterior descending (LAD) coronary artery, proximal 95% stenosis; diagonal, occluded proximal first diagonal artery; left circumflex coronary artery (LCX), occluded first marginal artery branch; and right coronary artery (RCA), mid-95% stenosis. All patients had no evidence of myocardial infarction and normal single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) at rest. From left to right, the images represent apical short axis, mid short axis, basal short axis, mid vertical long axis, and mid horizontal long axis. These patients show the typical distributions of perfusion defects associated with the specific coronary arteries involved.
Figure 21–13.
Stress single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) images demonstrating more complex patterns associated with known coronary lesions in patients with normal resting perfusion images and no history of prior myocardial infarction. Septal: Trapped septal perforator coronary artery in a patient with critical left anterior descending coronary artery (LAD) stenoses proximal and distal to the septal perforator takeoff and patent LAD internal mammary graft. Left circumflex coronary artery and right coronary artery (LCX+RCA): Occlusion of proximal LCX and proximal RCA. Left main (LM): Subtotal LM coronary artery stenosis; septal, septal perforator coronary artery.
Semiquantitative perfusion scoring systems standardize the visual interpretation of scans, reduce the likelihood of overlooking significant defects, and provide global indices for overall assessment of extent and severity of perfusion abnormality. Further, they are more systematic and reproducible than simple qualitative evaluation.
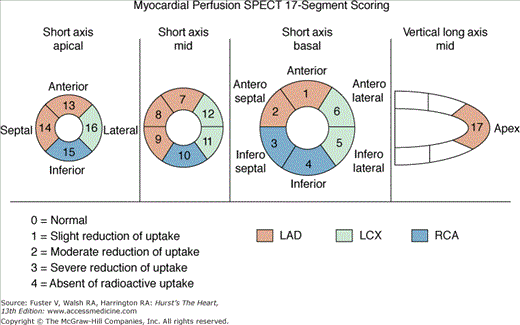
A 17-segment scoring system for SPECT MPI is preferred over the previously widely used 20-segment system because it more accurately represents the size of each segment (Fig. 21–14).48 Segmental assignment is based on three short-axis slices (four distal [apical], six mid, and six basal) representing the entire left ventricle (LV), with the apical segments visualized in a midventricular long-axis image. Each of the 17 segments has a distinct name and is scored using a 5-point system (0 = normal; 1, 2, 3 = mild [equivocal], moderate, and severe reductions of a radioisotope; and 4 = absence of detectable tracer uptake).2,31 Severe reversible perfusion defects with scores of 3 or 4 (see Fig. 21–12second row and bottom row; see Fig. 21–13second row and bottom row) can be reported as consistent with a critical (≥90%) coronary stenosis.31 The differences between the 17- and 20-segment models are that with 17-segment scoring, the smaller size of the distal short-axis slice is accounted for by four versus six segments and the apex is one rather than two segments.
Figure 21–14.
Diagrammatic representation of segmental division of the single-photon emission computed tomography (SPECT) slices and assignment of the segments to the individual coronary arteries using the 17-segment model. LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery.48
Segmental scoring systems lend themselves to the derivation of summed scores from the 17 to 20 segments (ie, global indices of perfusion).2,49 The overall extent and severity of perfusion defects is reflected by the summed stress score (SSS), the summed rest score (SRS), and the summed differences score (SDS), with the latter defined by SSS –SRS and measuring the degree of reversibility. Risk groups may be defined using SSS categories2,31 (Table 21–1).
| Summed stress score (SSS)a | Sum of the segmental scores at stress | Amount of infarcted, ischemic, or jeopardized myocardium |
| Summed rest score (SRS)a | Sum of the segmental scores at rest | Amount of infarcted or hibernating myocardium |
| Summed difference score (SDS) | SSS − SRS | Amount of ischemic myocardium |
| 20-Segment | 17-Segment | |
| Percent total | = SSS × 100/80 | = SSS × 100/68 |
| Percent ischemic | = SDS × 100/80 | = SDS × 100/68 |
| Percent fixed | = SRS × 100/80 | = SRS × 100/68 |
More recently, we have described expressing overall perfusion defects as percent myocardium involved (percent stress, reversible, fixed) and now routinely use this approach for clinical reporting and all prognostic publications.35 The conversion of summed scores to percent myocardium is accomplished by dividing the summed scores by the worst segmental score possible in the specific model used (68 for 17 segments, 80 for 20 segments) and multiplying by 100 (5-point scoring with 0 = normal and 4 = absent uptake). The benefits of this approach are that it provides a measure with intuitive implications (percent myocardium hypoperfused) not possible with the unit-less summed scores, it can easily be applied with scoring systems using varying numbers of segments (eg, 17, 20, 14, 12), and it is applicable to quantitative methods that directly measure these abnormalities as percent myocardium (see Table 21–1). In general, risk groups by the percent stress abnormal, which correlate with SSS risk groups, are as follows: <5%, normal or minimally abnormal; 5% to 10%, mildly abnormal; 11% to 14%, moderately abnormal; and ≥15%, severely abnormal.31,35,50 When converted to percent myocardium abnormal, the prognostic implications of the 17- and 20-segment scoring systems have been shown to be equivalent.51
A variety of commercial software packages are available to assist in image interpretation, and the quantitative measurements they can provide are summarized in Table 21–2. With respect to myocardial perfusion assessment, these computer approaches generally operate by automatic determination of the amount of radioactivity observed at rest and stress within each pixel or small zone of the myocardium, scaling this amount by the maximal amount of radioactivity in the myocardium (normalization), and then comparing this scaled amount to the lower limit of normal. The change between rest and stress is also assessed providing information about perfusion defect reversibility. With recent software developments, it is possible to automatically register and subtract the rest images from the stress images or serial stress or rest studies from previous studies, resulting in a “change” image. The change image reflects the amount of regional perfusion change.52 The results are most commonly displayed using polar maps. Figure 21–15 displays polar maps associated with the various scan abnormalities shown in Figs. 21–12 and 21–13.
| Type | Parameter | Comment |
|---|---|---|
| Perfusion | Perfusion defect extent | Expressed as % of LV myocardium |
| Perfusion defect severity | Related to the degree of hypoperfusion in the defect area | |
| Total perfusion deficit | Combines perfusion defect extent and severity; expressed as % of LV myocardial perfusion deficit | |
| Segmental scores and summed scores (poststress, rest, reversibility) | Depends on specific myocardial model chosen | |
| Normalized summed scores | Normalized to worse possible score in chosen model, therefore model independent | |
| Function (global) | LV ejection fraction | |
| LV end-systolic and end-diastolic volume | ||
| Peak filling rate (PFR), time to PFR | LV diastolic function | |
| Function (Regional) | Wall motion and wall thickening | |
| Other | LV contraction histogram | LV contraction phase/dyssynchrony |
| Lung-to-heart ratio | Ratio of uptake in lung and LV | |
| Transient ischemic dilation ratio | Ratio of LV cavity volume after stress vs at rest | |
| LV myocardial mass LV eccentricity, shape index | Estimates of LV shape (global or regional) |
Figure 21–15.
Quantitative stress polar maps of stress myocardial perfusion imaging (MPI) in patients shown in Figs. 21–12 and 21–13 showing typical vascular perfusion patterns; black represents regions of quantitative perfusion defect when compared to normal limit files. LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; LM, left main; RCA, right coronary artery.
Recent developments in quantitative analysis of SPECT MPI have been shown to provide greater accuracy in detecting CAD than expert visual assessment. Quantitative assessment of serial studies has also been shown to be more reproducible than expert visual assessment.53,110 These computer-based quantitative tools are often used in clinical practice as an adjunct to visual interpretation and are commonly used for SPECT MPI assessments in clinical trials. Because these computer-based quantitative programs do not take into account artifacts that may be easily detected visually (such as marked breast attenuation), the final interpretation currently always includes visual verification and, if necessary, modification.1,31 The results of quantitative analysis has been shown to be prognostically useful and may complement information derived from visual analysis alone.54
It is currently recommended that ECG gating also be performed with all SPECT protocols, whether 201Tl or 99mTc tracers are used.55 Gated SPECT MPI allows the assessment of a variety of ventricular function parameters. Regional wall motion or thickening is most commonly assessed by semiquantitative visual analysis, using the same segmental system used for perfusion defect assessment. Automatic computer-based methods quantify global function parameters, including left ventricular ejection fraction (LVEF), end-diastolic volumes (EDVS), end-systolic volumes (ESVs), and diastolic function. Regional LV myocardial wall motion and thickening can be quantified from gated SPECT MPI images and is useful in the identification of hibernation, stunning, and infarction as well as distinguishing true perfusion defects from attenuation artifacts. Also, LV dyssynchrony can be quantified by measuring the exact time of contraction of each myocardial sample point relative to the cardiac cycle; this is done by taking the first Fourier harmonic of its temporal displacement curve, the phase angle of which forms the basis for all of the synchrony measurements.56 Phase information from all LV sample points can be pooled together into a phase histogram, whose parameters (histogram standard deviation, bandwidth, and entropy) offer a global measure of intraventricular dyssynchrony. Moreover, regional contraction differences (for example, between the lateral and the septal LV wall) can be measured, similarly to what is done in echocardiography.56-58 Gating of both the rest and the poststress acquisition is currently performed in most laboratories, providing the ability to detect stress-induced stunning by finding of regional wall motion abnormalities after stress not seen on the resting studies.18
Quantitation of ventricular function and volumes with gated SPECT MPI can be performed by a variety of algorithms.59-61 The most common approaches are fully 3D and based on the automatic detection of endocardial and epicardial surface points.62-64 In validation studies of LVEF by gated SPECT published to date,1 the agreement between gated SPECT and other standard measurements of LVEF has been shown to be very good to excellent. Although LVEF measured by the various quantitative algorithms correlate highly, the thresholds for abnormality are different depending on the method used.65,66 With some methods, the normal threshold for the global LVEF measured by gated SPECT MPI images is slightly lower than that measured using other imaging modalities (~45%), principally because of the use of only eight gating intervals.1 Recently, 16-frame gating has become more common, reducing the underestimation of LVEF. Sex-based normal limits for LVEF and LV volumes and volume indices have been reported.67 With multidetector systems, LVEF and LV volumes by gated 201Tl SPECT MPI correlate highly with those of 99mTc-sestamibi SPECT MPI.1
In addition to perfusion defects, several nonperfusion abnormalities can be observed with SPECT MPI, including size and shape of the LV, transient ischemic dilation (TID) of the LV,68,69 RV myocardial uptake pattern,70 RV size, and abnormalities of lung uptake or other abnormal extracardiac activity. TID is considered present when the LV cavity appears to be significantly larger in the poststress images than at rest68,69 and may actually be an apparent cavity dilation secondary to diffuse subendocardial ischemia (obscuring the endocardial border). This explains why TID may be seen for several hours following stress, when true cavity dilation is probably no longer present. The correlation between LV TID and lung uptake is weak, suggesting that there may be different pathophysiologic mechanisms for each, and their measurements may be complementary in assessing the extent and severity of CAD for risk stratification.71 TID is considered to represent severe and extensive ischemia and has been shown to be highly specific for critical stenosis (>90% narrowing) in vessels supplying a large portion of the myocardium (ie, proximal left anterior descending or multivessel 90% lesions).68,69 Dipyridamole or adenosine-induced TID has similar implications as those associated with exercise.72 Figure 21–16 illustrates an example of TID on SPECT MPI from a patient with severe three-vessel CAD. TID can easily be measured by the quantitative gated SPECT algorithms. The upper limit of normal for the TID ratio with on-stress SPECT MPI depends on the stress type and the imaging protocol used.73 With exercise stress, the upper limit of normal for TID has been reported to be 1.22 for dual-isotope imaging and 1.14 for 2-day 99mTc imaging.69 For reasons that are not known, with vasodilator stress, the upper limit of normal to consider TID to be present is higher, approximately 1.36 with dual-isotope SPECT.72,74
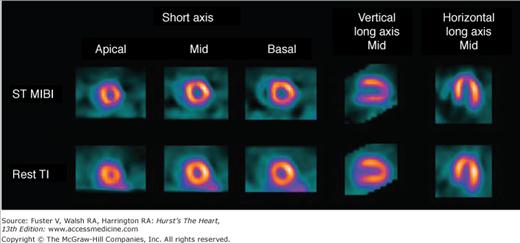
Figure 21–16.
Exercise myocardial perfusion single-photon emission computed tomography (SPECT) in a 67-year-old diabetic woman with shortness of breath and no known coronary artery disease. The imaging shows normal perfusion and transient ischemic dilation (ratio, 1.40). Invasive coronary angiography revealed severe three-vessel disease (70%-90% stenosis). ST MIBI, stress technetium-99m–sestamibi; Tl, thallium-20.
Increased lung uptake of thallium reflects increased pulmonary capillary wedge pressure. Nonischemic causes of increased pulmonary capillary wedge pressure, such as mitral regurgitation and mitral stenosis, are also associated with increased pulmonary thallium uptake. Increased thallium lung uptake after exercise has been shown to have incremental prognostic information over myocardial perfusion defect assessment.75 Prognostic value of increased pulmonary uptake of 99mTc-sestamibi has recently been reported.76
A number of patient characteristics or stress MPI findings have been found to be associated with increased patient risk of adverse events (Table 21–3). Clinical high-risk markers tend to be related to patient age, rest ECG characteristics, diabetes mellitus, presenting symptoms, and prior CAD. Stress test markers of risk include stress-related BP and HR responses, ECG changes, and functional response. MPI high-risk markers include the presence of extensive ischemia or scar (or its surrogate) and the presence of significant LV dysfunction. Hence, for any MPI result, the estimated risk of the patient should be increased in proportion to the number of higher-risk markers present. This is especially the case in patients found to have normal stress perfusion and function, but with TID, LV enlargement, or severe ST-segment changes.
| Clinical and Demographic | Stress Test | SPECT |
|---|---|---|
Diabetes mellitus (especially insulin dependence, more so in women) Marked resting ECG abnormalities: atrial fibrillation, LBBB, RBBB, other block (first or second degree, hemiblocks), LVH with repolarization, PVCs Increasing age Increased rest HR Dyspnea as presenting symptom Typical angina Presentation with unstable angina History of prior CAD (especially prior MI) | Brief exercise duration Severe ST-segment depression Need for pharmacologic stress Exercise-induced hypotension High Duke treadmill score Stress-induced ventricular dysrhythmias Tachycardic response to stress Low HR reserve achieved Blunted HR response to adenosine Nonwalking vasodilator stressa | TID Lung uptake Stress-induced stunning Reduced EF Severe and/or extensive defects Increased RV tracer uptake Akinetic-dyskinetic LV segments LV enlargement |
Chronotropic incompetence, defined as a low percentage of HR reserve achieved [(peak HR –rest HR)/(220 –age –rest HR) × 100], with <80% considered abnormal, has been shown to be a powerful predictor of cardiac death and all-cause mortality in patients undergoing exercise SPECT MPI.77,78 In a recent study, inability to achieve 80% of HR reserve was a more powerful predictor of cardiac death than failure to reach 85% of MPHR.78 Also, patients with normal SPECT MPI but abnormal HR reserve achieved were at just as high a risk for overall mortality as patients with abnormal SPECT MPI but normal HR reserve achieved. Thus, physicians should not be misled that patients with normal stress SPECT MPI in the setting of an abnormal HR reserve are a low-risk group—they are at substantial risk. It has been suggested that when assessing HR response to exercise, the HR reserve approach should be used and should replace the traditional percentage of age-predicted HR achieved.
As with any diagnostic test, quality control is critical to clinical application of SPECT MPI. Artifactual perfusion defects have a variety of causes, the most common of which are patient motion, breast and diaphragmatic attenuation, reconstruction artifacts caused by adjacent or superimposed extracardiac radioactivity, and poor count statistics.55 With gated SPECT MPI, several technical artifacts can affect the accuracy of LVEF measurement. LVEFs can be overestimated in analyzing gated SPECT images of small hearts66 because of the limitations of spatial resolution with SPECT. In patients with left ventricular hypertrophy (LVH), LVEF may be underestimated because of failure to accurately determine the endocardial border in the presence of the large muscle mass. Marked arrhythmia results in a falsely low LVEF measurement.
Assessment of myocardial viability with the myocardial perfusion tracers applies to segments with contractile dysfunction.31 Viability is considered present if the degree of uptake at rest, redistribution, or following nitrate-augmented rest injection79 is normal or nearly normal. Various patterns of myocardial viability associated with regional wall motion abnormality in these studies are shown in Fig. 21–11. A dysfunctional segment or region with severely reduced or absent uptake of radioactivity on a viability study is considered to be nonviable. Areas with moderate reduction of counts in these conditions (eg, a score of 2/4 at redistribution or nitrate-augmented rest) are usually partially viable, and patients in this group have a variable response in terms of improvement after revascularization. When perfusion defects are seen at rest and not after redistribution or after separate reinjection following administration of nitroglycerin, SPECT MPI is commonly interpreted as showing resting ischemia and considered to represent regions of critically reduced blood flow to viable myocardium,38 generally implying the need for consideration of rapid revascularization.
Clinical Applications of SPECT MPI
The principles underlying the efficient use of stress nuclear techniques and the optimal use of the test results are discussed below. The most common applications are assessing the likelihood of CAD in patients with suspected disease, assessing the likelihood that a patient with known CAD has ischemia, and assessing the magnitude of ischemia for prognostic purposes. The section that follows explores these applications and discusses the evidence for the use of SPECT MPI in specific patient populations commonly encountered in clinical cardiology settings.
Central to appropriate patient selection for nuclear imaging and the interpretation of test results is the ability to determine an individual patient’s pretest likelihood of CAD based on demographic, clinical, and historical information. The accurate evaluation of pretest likelihood of CAD allows for the appropriate selection of patients who would most likely benefit from referral to nuclear imaging. The pretest likelihood or risk assessment may be estimated from published nomograms or by using available computerized programs (see Chaps. 16 and 64). Several models have been developed to predict significant and extensive CAD as well as cardiac survival.80
The rationale for applying SPECT MPI to noninvasive diagnostic testing is based on Bayesian theory (see Chaps. 16 and 64), by which for a given test result, the posttest likelihood is a function of only three variables: the patient’s pretest likelihood of disease and the test’s sensitivity and specificity. The degree to which the test result alters the posttest likelihood is strongly affected by the pretest likelihood of disease. For any imperfect test, the greatest shift in posttest likelihood of disease occurs in patients with an intermediate pretest likelihood of CAD. As a benchmark, for a test with sensitivity and specificity of 90%, a patient with a pretest likelihood of 50% (eg, a 50-year-old man or a 65-year-old woman with atypical angina81) will have a posttest likelihood of 10% (low) with a normal test result and a 90% likelihood (high) with an abnormal test result. For purposes of assessing the posttest likelihood of CAD when using SPECT MPI, the patient’s pretest likelihood takes into account all available information, including age, sex, symptoms, risk factors, the degree of coronary atherosclerosis if known (eg, from a coronary artery calcium [CAC] score), and the results of the nonnuclear stress testing components of the examination (eg, the duration of exercise and degree of ST-segment depression).
Detection of CAD is one of the most common indications for performing stress SPECT MPI. This referral is most appropriate in patients who have an intermediate likelihood of CAD. Exercise is the preferred form of stress because of the additional information derived from clinical, hemodynamic, and ECG responses to exercise. As noted earlier, pharmacologic vasodilator stress is preferred in patients with LBBB or paced LV rhythms (class I indication in guidelines2) and patients with moderate aortic stenosis.
The accuracy of diagnostic testing for CAD is defined clinically based on sensitivity and specificity for identification of angiographically significant stenoses, most commonly using either a 50% or 70% diameter-narrowing cutoff. Recent ACC/AHA/ASNC guidelines on cardiac radionuclide imaging report a pooled sensitivity and specificity of 87% and 73%, respectively, for exercise SPECT MPI (based on 33 published studies) and 89% and 75%, respectively, for vasodilator SPECT MPI (based on 17 published studies) for detection of CAD.2 Improved predictive accuracy of nuclear testing over pretest information and ECG stress testing has been consistently documented.82 In women, the specificity of SPECT MPI is increased with gated 99mTc-based agents compared with ungated 201Tl SPECT MPI, which is attributed to less susceptibility to breast attenuation of gated SPECT MPI with 99mTc-based agents.83 The ability to immediately reacquire SPECT images with 99mTc-based agents when either attenuation or motion artifact is suspected further increases specificity with these agents.16,84 Improvements in specificity have also been reported with the use of attenuation correction algorithms.85 The use of ECG gating attenuation correction and combined prone and supine imaging are also considered to be associated with improvement in reader confidence.
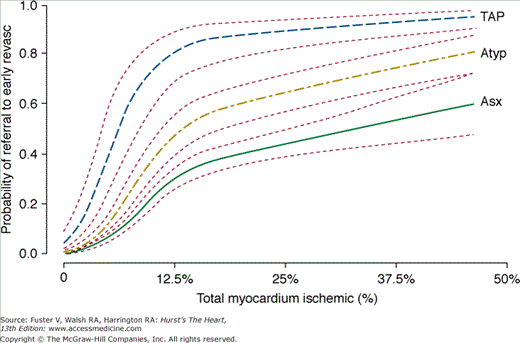
A major limitation in assessing the diagnostic accuracy of SPECT MPI for the detection of CAD is the current clinical reality that the decision to perform catheter-based coronary angiography, the gold standard, is determined by the MPI result, thereby biasing the population available for the analysis of test accuracy. Using a multivariable analysis, Hachamovitch and colleagues35 have demonstrated that post-SPECT referral to catheterization was predominantly driven by the amount of MPI-determined inducible ischemia reported (83% of all information) (Fig. 21–17), with related factors (anginal symptoms, ischemic ECG) appearing to also influence this decision. Thus, in estimating the true sensitivity and specificity of noninvasive testing, this bias (partial verification bias) must be taken into account.86,87 Because routine patient workup results in preferential catheterization of patients with abnormal (ischemic) test results, this referral bias leads to an overestimation of test sensitivity and a reduction in test specificity,88 with the latter showing the most dramatic change. Indeed, in the extreme case, test sensitivity and specificity would approach 100% and 0%, respectively. For example, Miller and colleagues89 reported that uncorrected SPECT specificity was <10% in men.
Figure 21–17.
Relationship between percent myocardium ischemic and probability of referral to early revascularization (Revasc; <60 days post–SPECT MPI). Results are based on multivariable modeling in 10,647 patients. In this study, percent myocardium ischemic was most strongly associated with referral to revascularization (83% of all information used for decision making). Furthermore, patients’ presenting symptoms also influenced this process as evidenced by greater likelihood of referral at any level of ischemia with typical versus atypical versus asymptomatic patients. Atyp, atypical; Asx, asymptomatic; MPI, myocardial perfusion imaging; SPECT, single-photon emission computed tomography; TAP, typical angina pectoris. With permission from Hachamovitch et al.35
The normalcy rate has been advocated as another means of assessing test specificity without requiring the angiographic standard.88 The normalcy rate is defined as the percentage of patients with normal test results in a population with a low likelihood of disease, usually using a <5% criterion. The 2003 ACC/AHA/ASNC guidelines reported a normalcy rate for SPECT MPI of 91%. Even in obese patients, normalcy rates >90% have been reported when attenuation correction or combined supine/prone imaging is used.16,90 This approach, however, is not without limitations, because patients with a very low likelihood of CAD are so much “healthier” than the patients with whom they are being compared.
SPECT MPI is not recommended for use as a screening test. For CAD diagnosis, the principal application is in patients with an intermediate likelihood of CAD.2,82,91-93 In the lower range of intermediate CAD likelihood (eg, 0.15-0.50), many advocate the use of exercise tolerance test (ETT) alone. Although patients with pre-ETT likelihood of CAD in the 0.50 to 0.85 range could also be considered candidates for ETT alone,2,91 because a negative ETT would not result in a low CAD likelihood, many experts consider SPECT MPI (or coronary CT angiography) the appropriate first test. Patients with an indeterminate CAD likelihood after ETT (eg, intermediate-risk Duke treadmill score2,92) are candidates for SPECT MPI. Patients with ECG uninterpretable for ETT (eg, LVH, digoxin, Wolff-Parkinson-White, >1-mm resting ST-segment depression, LBBB, permanent pacemaker) are candidates for SPECT MPI rather than ETT. As noted, the development coronary CT angiography may alter this approach, possibly becoming the first diagnostic test toward the lower part of the pretest likelihood spectrum.
A widely used paradigm in patient management is that of a risk-based approach to patients with suspected CAD in whom symptoms are nonlimiting. In patients referred directly to catheterization for any reason, precatheterization SPECT MPI may serve to identify the culprit lesion. However, in less symptomatic patients, precatheterization risk assessment is more important. With a risk-based approach, the focus is not on predicting the presence of CAD but on identifying patients at risk for specific, potentially preventable adverse events. Subsequent management focuses on reducing the risk of these outcomes, whether cardiac death, nonfatal myocardial infarction (MI), or CAD progression. Invasive diagnostic and therapeutic procedures are limited to patients who are most likely to benefit from them. The basic concept underlying the use of nuclear testing for risk stratification is that patients known to be at high or low risk for events would not need risk stratification with nuclear imaging because they are already stratified.
For the purposes of risk assessment, it has been proposed that low risk be defined as a <1% annual cardiac mortality rate and intermediate risk be defined by the range of 1% to 3% per year.91 Because the mortality risk for patients undergoing revascularization is ≥1% (see Chaps. 62 and 65), symptomatic patients with a <1% annual mortality risk would not appear to be candidates for revascularization to improve survival. It has been suggested that a >3% annual mortality risk is a threshold to identify patients with symptoms whose mortality rate can be improved by coronary artery bypass surgery (CABS).94 SPECT MPI is most appropriate in patients with >1% annual mortality and intermediate or high likelihood of CAD or with known CAD. The exact level of these risk thresholds would vary according to the population being tested.
Many major determinants of prognosis in CAD are assessed by measurements of stress-induced perfusion and function, including infarcted and jeopardized myocardium (supplied by vessels with hemodynamically significant stenosis) and the degree of jeopardy (tightness of the individual coronary stenosis). Of additional importance is the stability (or instability) of the CAD process, a factor that may explain an apparent paradox. Although nuclear tests in general are expected to identify only hemodynamically significant stenoses, it has been observed that most MIs occur in regions with <50% diameter narrowing.95 Yet normal SPECT MPI, expected in patients with no or insignificant CAD, is associated with a low risk of either cardiac death or nonfatal MI. Several explanations may account for this apparent paradox. Patients with severe CAD may have more numerous mild plaques subject to potential instability and rupture than patients with no severe stenoses. Furthermore, a paradoxical vasoconstrictive response to stress caused by endothelial dysfunction with unstable mild stenoses may also contribute.7
Stress SPECT MPI is an integral part of the evaluation of symptomatic patients for CAD and is commonly used in patients at intermediate post-ETT risk or with resting ECG abnormalities. As with diagnostic testing, if the patient’s rest ECG is uninterpretable for purposes of stress testing, direct referral to SPECT MPI is effective in prognostic stratification (see Chap. 16).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree