Although many clinical trials and meta-analyses have demonstrated that lower serum low-density lipoprotein cholesterol (LDL-C) levels are associated with proportionately greater reductions in the risk of cardiovascular disease events, not all patients with hypercholesterolemia are able to attain risk-stratified LDL-C goals with statin monotherapy. Elucidation of the pathophysiology of genetic disorders of lipid metabolism (e.g., familial hypercholesterolemia) has led to the development of several novel lipid-lowering strategies, including blocking the degradation of hepatic LDL-C receptors that are important in LDL-C clearance, or the inhibition of apoprotein synthesis and lipidation. Mipomersen and lomitapide are highly efficacious new agents available for the treatment of patients with homozygous familial hypercholesterolemia. The recent introduction of PCSK9 inhibitors (alirocumab and evolocumab) have made it possible for many patients to achieve very low LDL-C concentrations (e.g., <40 mg/dl) that are usually not attainable with statin monotherapy. Ongoing clinical trials are examining the impact of very low LDL-C levels on cardiovascular disease event rates and the long-term safety of this approach.
Over the last 2 decades, guidelines on the management of low-density lipoprotein cholesterol (LDL-C) in patients for cardiovascular risk reduction have emphasized lower LDL-C targets, greater reduction for high-risk patients, and expansion of lipid lowering to larger numbers of patients. A growing body of evidence has demonstrated that lower posttreatment LDL-C levels are associated with proportionately greater reductions in the risk of cardiovascular disease (CVD) events. For example, a meta-analysis of large, long-term, randomized clinical trials of lipid-lowering therapy that was performed by the Cholesterol Treatment Trialists Collaboration found that each reduction in serum LDL-C concentration of 1.0 mmol/L (38.6 mg/dl) decreased the annual rate of CVD events by about 1/5. In the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) study, which examined the combination of ezetimibe and statin therapy in patients who were hospitalized after acute coronary syndrome, the addition of ezetimibe to a statin produced greater LDL-C lowering than statin therapy alone, which was associated with a significant incremental reduction in the risk of CVD events. Post hoc analyses from the Pravastatin or Atorvastatin Evaluation and Infection Therapy, Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin, and the Treating to New Targets studies all support the conclusion that lower LDL-C levels result in greater CVD end point reduction. However, despite the clear reduction in CVD events with more aggressive lipid-lowering therapy, not all patients attain risk-stratified LDL-C goals with statin monotherapy. Approximately 10% to 20% of patients develop statin intolerance, and the discontinuation of statin therapy is common in clinical practice. Some patients require adjuvant therapy with ezetimibe, bile acid-binding resins, and other medications to facilitate LDL-C reduction. Poor adherence to statin therapy is also common and is associated with a significant reduction in the effectiveness of statin therapy for CVD risk reduction.
Lipoproteins are complex particles that consist of a central core containing cholesterol esters and triglycerides, which is surrounded by free cholesterol, phospholipids, and apolipoproteins. Apolipoprotein B (ApoB) is the major lipoprotein structural component of very low-density lipoprotein (VLDL), intermediate density lipoprotein (IDL), and LDL. LDL particles (LDL-P) represent the end product of VLDL catabolism. Lipoprotein lipase hydrolyzes the triglycerides in VLDL, which results in the progressive formation of IDL and then LDL-P.
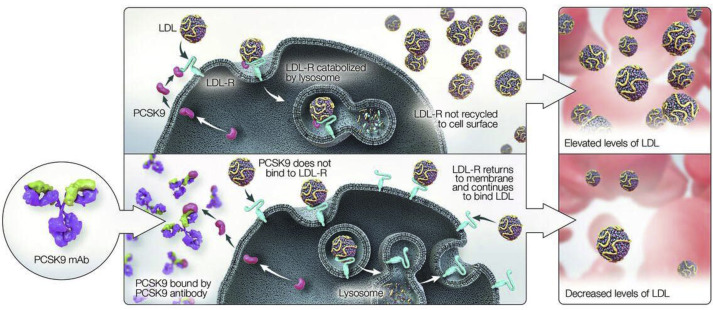
Circulating LDL-C is cleared from the circulation primarily by hepatic low-density lipoprotein receptors (LDL-Rs; they can also be removed by the LDL-R–related protein). An important part of this process is the recycling of LDL-Rs to the hepatocyte surface after intracellular uptake. It has been estimated that each LDL-R typically recirculates to the hepatocyte surface approximately 150 times. ApoB acts as a ligand that binds circulating LDL-C particles to LDL-Rs on the hepatocyte surface, and the resulting LDL/LDL-R complex is internalized into the hepatocyte, thereby removing LDL from the circulation. In order for the LDL-R/LDL-P complex to undergo uptake into hepatocytes, they must concentrate in clathrin-coated pits along the hepatocyte membrane, a process potentiated by LDL-R adaptor protein-1 (LDL-RAP1). Loss-of-function polymorphisms in LDL-RAP1 lead to hypercholesterolemia secondary to reduced LDL-P uptake, a condition known as autosomal recessive hypercholesterolemia. Subsequent to being concentrated in clathrin-coated pits, LDL-P/LDL-R complexes are enveloped into endosomal vesicles, which enter the cytosol of hepatocytes. The endosomal vesicle subsequently fuses with a lysosome, the LDL-P/LDL-R complex dissociates, and the LDL-P is catabolized whereas LDL-R is recycled to the cell surface to initiate another round of LDL-P uptake. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a molecule that regulates expression of the LDL-R. The LDL-R/LDL-P complex binds PCSK9, which targets the complex for proteolytic destruction in the lysosome. If the LDL-R/LDL-P complex does not bind PCSK9, once the LDL-P dissociates from LDL-R within the hepatocyte cytosol, it gets recycled to the cell surface to reinitiate another cycle of LDL-P uptake. Increased expression of PCSK9 leads to reduced surface expression of LDL-R and large elevations in LDL-C and LDL-P.
Familial hypercholesterolemia (FH) is a genetic disorder that is characterized by abnormal lipid metabolism, markedly elevated serum LDL-C concentration, and increased risk of premature atherosclerotic disease. FH is caused by mutations in the genes that encode LDL-R, ApoB, PCSK9, or LDL-RAP1. These mutations cause defective LDL-R function, reduced affinity between ApoB and LDL-R, deficiency in adaptor protein-1, and increased activity of PCSK9, which result in decreased binding or elimination of circulating LDL-P and elevated serum LDL-C concentrations.
Heterozygous FH (HeFH) is defined clinically by an LDL-C of ≥160 mg/dl for children and ≥190 mg/dl for adults, with 1 first-degree relative similarly affected or with premature coronary artery disease (CAD); or by positive genetic testing for an LDL-C–raising gene defect (LDL-R, ApoB, or PCSK9). Homozygous FH (HoFH) is usually characterized by no or very low levels of expression of functional LDL-Rs on the surface of hepatocytes and is defined clinically by an LDL-C level of ≥400 mg/dl and a history of FH in 1 or both parents. Patients with HoFH experience severe elevations in risk for premature CAD and can become symptomatic by the second or third decade of life, making early diagnosis extremely important. Patients with FH can present with physical stigmata which may include xanthomas, xanthelasmas, heart murmurs, aortic outflow obstruction and heart failure, and premature peripheral and carotid arterial disease. Cascade screening targets the relatives of patients with established FH and is cost-effective.
Elucidation of the genetic and molecular mechanisms of FH has led to the development of several treatment strategies for FH and other causes of hypercholesterolemia. Some of these strategies include blocking the activity of PCSK9, preventing the synthesis of ApoB, or blocking the lipidation of ApoB that is essential to produce VLDL-C.
Monoclonal Antibodies Against PCSK9
PCSK9 plays a key part in the regulation of LDL-C levels and has recently emerged as a critical therapeutic target for patients with hypercholesterolemia. PCSK9 is a serine protease that is expressed in various tissues such as the liver, brain, kidney, small intestine, and colon, with the highest levels occurring in the liver. It is synthesized primarily as a 692 amino acid precursor of approximately 74 kDa (pro-PCSK9). Autocatalytic cleavage in the endoplasmic reticulum generates a 14-kDa prodomain and a 60-kDa catalytic fragment, which are secreted into the systemic circulation. The catalytic subunit of PCSK9 binds extracellularly to the epidermal growth factor-like domain of the LDL-R, is internalized into the cell, and chaperones LDL-R to the lysosome for degradation. This process prevents recycling of LDL-Rs back to the hepatocyte surface, reducing LDL-C uptake, and increasing plasma LDL-C levels.
Human genetic studies have demonstrated that mutations of PCSK9 significantly affect LDL-C levels and CVD risk. Gain-of-function mutations of PCSK9 result in increased lysosomal degradation of LDL-R, fewer LDL-Rs on hepatocyte surfaces, less LDL-C uptake, and higher levels of circulating LDL-C. In contrast, PCSK9 loss-of-function mutations result in more LDL-Rs on the hepatocyte surface and greater uptake of serum LDL-C. Loss-of-function mutations are considered to occur in approximately 1% to 3% of the population and have been associated with decreases of LDL-C concentrations of approximately 15% to 40%, as well as a reduction of coronary heart disease (CHD) events of 47% to 88%. These observations suggest that pharmacologic inhibition of PCSK9 activity might provide a novel approach to lowering LDL-C levels in patients with hypercholesterolemia.
Fully human monoclonal antibodies have been developed to bind PCSK9 and induce steric hindrance so that PCSK9 cannot bind to the LDL/LDL-R complex. This increases recycling of the LDL-R to the hepatocyte surface, promotes clearance of LDL-C, and lowers serum LDL-C concentration. The effects of monoclonal antibody inhibition of PCSK9 are illustrated in Figure 1 . The clinical development of PCSK9 inhibition as a therapeutic strategy for hypercholesterolemia occurred very rapidly over the last decade. The initial publications describing the effects of PCSK9 on lipid metabolism appeared in 2003, and by 2012, the beneficial effects of pharmacologic PCSK9 inhibition using monoclonal antibodies had been reported in randomized clinical trials of patients with elevated cholesterol levels. Preclinical and initial clinical studies demonstrated that monoclonal antibodies against PCSK9 produce rapid and sustained clearance of unbound systemic PCSK9, and that this suppression of PCSK9 activity persisted for several weeks after a single injection. In phase 2 studies, approximately 70% to 90% of patients with hyperlipidemia despite statin therapy were able to achieve LDL-C concentrations of ≤70 mg/dl. Several PCSK9 inhibitors are being evaluated in phase 2 and phase 3 clinical trials, and 2 have recently been approved for the treatment of patients with hypercholesterolemia. Alirocumab was approved by the US Food and Drug Administration on July 24, 2015, and is indicated, in addition to diet and maximally tolerated statin therapy, for adult patients with HeFH or patients with clinical atherosclerotic CVD who require additional lowering of LDL-C. On August 27, 2015, evolocumab was approved by the Food and Drug Administration, in addition to diet and maximally tolerated statin therapy, for use in adult patients with HeFH, HoFH, or clinical atherosclerotic CVD who require additional lowering of LDL-C. Large clinical trials have demonstrated that both agents significantly reduce atherogenic lipoprotein burden in serum and may reduce the risk for cardiovascular events, compared with the usual standard of care.
Alirocumab
In phase 1 clinical trials, intravenous or subcutaneous (SC) administration of alirocumab produced dose-dependent reductions of serum LDL-C in healthy control subjects, with decreases of approximately 60% to 70% from baseline levels at the highest doses. In a randomized, double-blind, placebo-controlled, 12-week clinical trial of patients with LDL-C of ≥100 mg/dl on stable atorvastatin therapy, SC administration of alirocumab decreased mean LDL-C concentration by 39.6%, 64.2%, and 72.4% at alirocumab doses of 50 mg, 100 mg, and 150 mg every other week, respectively, compared with a mean decrease of 5.1% with placebo (p <0.0001 for each alirocumab dose group vs placebo). Administration of alirocumab at doses of 200 mg or 300 mg every 4 weeks also significantly reduced LDL-C concentrations from baseline, but with greater variability over time. The addition of alirocumab to atorvastatin therapy also improved several other lipid measures, including total cholesterol, ApoB, non–high-density lipoprotein cholesterol (non–HDL-C), HDL-C, and triglycerides. Alirocumab produced a dose-related decrease in LDL-C concentrations in a phase 2 clinical trial of adults with HeFH who were receiving stable statin therapy, with a mean reduction of LDL-C concentration of 67.9% between baseline and 12 weeks at the highest dose (150 mg every other week).
Alirocumab is being evaluated in the ODYSSEY clinical research program, which consists of 14 clinical trials that have enrolled more than 23,500 patients with hypercholesterolemia at more than 2000 study centers around the world. Two randomized, double-blind, placebo-controlled clinical trials (the Efficacy and Safety of Alirocumab vs Placebo on Top of Lipid-Modifying Therapy in Patients with Heterozygous Familial Hypercholesterolemia not Adequately Controlled with Their Lipid-Modifying Therapy [ODYSSEY FHI] trial and the Study of Alirocumab in Patients with HeFH Who Are not Adequately Controlled with their Lipid-Modifying Therapy [ODYSSEY FHII trial]) examined the safety and efficacy of alirocumab as add-on therapy with statins in patients with HeFH who had inadequate LDL-C control despite maximally tolerated statin treatment with or without other lipid-lowering therapy. In the ODYSSEY FHII study, LDL-C decreased by a mean of 48.8% from baseline in the alirocumab group and increased by a mean of 9.1% in the placebo group (percent difference for alirocumab vs placebo, 57.9%; p <0.0001). In the ODYSSEY FHII study, mean LDL-C decreased by 48.7% for the alirocumab group and increased by 2.8% in the placebo group (percent difference for alirocumab vs placebo, 51.4%; p <0.0001).
Two additional randomized, double-blind, clinical trials (the Efficacy and Safety of Alirocumab vs Placebo on Top of Lipid-Modifying Therapy in Patients with High Cardiovascular Risk and Hypercholesterolemia [ODYSSEY COMBO I] and the Efficacy and Safety of Alirocumab vs Ezetimibe on Top of Statin in High Cardiovascular Risk Patients with Hypercholesterolemia [ODYSSEY COMBO II]) examined the safety and efficacy of alirocumab as add-on therapy with statins, with or without other lipid-lowering drugs, in patients with high cardiovascular risk and suboptimal control of hypercholesterolemia despite maximally tolerated statin therapy. In the ODYSSEY COMBO I trial, patients were randomized to alirocumab 75 mg (n = 209) or placebo (n = 107) SC every other week in addition to statin therapy. The alirocumab dose could be increased to 150 mg at week 12 if LDL-C concentration remained at ≥70 mg/dl. In the ODYSSEY COMBO II trial, patients were randomized to alirocumab 75 mg SC every other week (plus oral placebo) or oral ezetimibe 10 mg daily (plus SC placebo) in addition to statin therapy. In the ODYSSEY COMBO I trial, the mean LDL-C at 24 weeks (the primary end point) decreased by 48.2% in the alirocumab group and by 2.3% in the placebo group (mean difference, 45.9%; p <0.0001). In the ODYSSEY COMBO II trial, the mean LDL-C at 24 weeks (again, the primary end point) decreased by 50.6% with alirocumab versus 20.7% with ezetimibe (difference, 29.8%; p <0.0001).
The Efficacy and Safety of Alirocumab vs Placebo on Top of Lipid-Modifying Therapy in Patients with Heterozygous Familial Hypercholesterolemia (ODYSSEY HIGH FH) study examined the efficacy of alirocumab in patients with HeFH on maximal statin therapy and an LDL-C concentration of ≥160 mg/dl at baseline. Patients continued statin therapy and were randomized to treatment with alirocumab 150 mg SC every other week (n = 72) or placebo (n = 35). After 4 weeks, LDL-C concentration decreased by a mean of 45.7% from baseline with alirocumab versus 6.6% with placebo (difference, 39.1%; p <0.0001). LDL-C concentration of <100 mg/dl was achieved by 57% of patients in the alirocumab group versus 11% of patients in the placebo group (p <0.0001).
Finally, longer term outcomes (up to 78 weeks) were examined in patients with or without HeFH in the Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia not Adequately Controlled with their Lipid Modifying Therapy (ODYSSEY LONG TERM) study. Patients with HeFH (31% of the study population), established CHD or CHD risk equivalents (e.g., peripheral artery disease, stroke, chronic kidney disease, diabetes mellitus; 69% of the study population), and who had LDL-C levels of ≥70 mg/dl while receiving statins at the maximum tolerated dose continued statin therapy and were randomly assigned to additional treatment with double-blind alirocumab 150 mg (n = 1,553) or placebo (n = 788) by SC injection every 2 weeks for 78 weeks. At week 24, LDL-C decreased by a mean of 61.0% for the alirocumab group and increased by 0.8% in the placebo group, for a between-group difference of 61.9% (p <0.001). Similar improvements in patients’ LDL-C were noted for those with or without HeFH. Alirocumab also produced greater reductions than placebo for several other lipid variables, including non–HDL-C, ApoB, total cholesterol, lipoprotein(a), and triglycerides ( Table 1 ). At the end of 78 weeks, LDL-C decreased by a mean of 52% for patients randomized to alirocumab and increased by 3.6% among patients in the placebo group ( Figure 2 ). Cardiovascular events were noted for 4.6% of patients in the alirocumab group and for 5.1% of patients in the placebo group. In a post hoc analysis, the combined incidence of major adverse events (death from CHD, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) was significantly lower for the alirocumab group than for the placebo group (1.7% vs 3.3%; relative risk reduction, 48%; p = 0.02).
| Percent change from baseline to Week 24 in lipid variables | Alirocumab (N = 1530) | Placebo (N = 780) | Least-squares mean difference | 95% CI | P Value |
|---|---|---|---|---|---|
| Non-HDL cholesterol | -51.6 ± 0.6 | 0.7 ± 0.9 | -52.3 ± 1.1 | -54.4 to -50.2 | <.001 |
| Apolipoprotein B | -52.8 ± 0.7 | 1.2 ± 1.0 | -54.0 ± 1.2 | -56.3 to -51.7 | <.001 |
| Total cholesterol | -37.8 ± 0.5 | -0.3 ± 0.7 | -37.5 ± 0.8 | -39.1 to -35.9 | <.001 |
| Lipoprotein(a) | -29.3 ± 0.7 | -3.7 ± 1.0 | -25.6 ± 1.3 | -28.1 to -23.1 | <.001 |
| Fasting triglycerides | -15.6 ± 0.8 | 1.8 ± 1.2 | -17.3 ± 1.4 | -20.1 to -14.6 | <.001 |
| HDL cholesterol | 4.0 ± 0.4 | -0.6 ± 0.5 | 4.6 ± 0.7 | 3.3 to 5.9 | <.001 |
| Apolipoprotein A1 | 4.0 ± 0.4 | 1.2 ± 0.6 | 2.9 ± 0.7 | 1.6 to 4.2 | <.001 |

Evolocumab
In a phase 2 clinical trial, patients with an LDL-C of ≥100 mg/dl and <190 mg/dl who were not currently receiving statin treatment were randomized to receive placebo or one of several doses of evolocumab (70, 105, or 140 mg SC every other week; or 280, 350, or 420 mg every 4 weeks). After 12 weeks, each of the evolocumab dose regimens was associated with a significantly greater reduction in LDL-C than placebo, achieving a mean reduction from baseline of 50.9% with the 140 mg every 2 weeks regimen, and a reduction of 48.0% with the 420 mg every 4 weeks regimen. Evolocumab has also been shown to significantly lower LDL-C concentrations as monotherapy in patients who were intolerant to statin therapy and to reduce serum concentrations of lipoprotein(a). A recent pooled analysis of data from 4 phase 2 clinical trials with a combined population of 1,359 patients found that evolocumab reduced mean lipoprotein(a) concentrations by approximately 25% to 30% compared with placebo after 12 weeks.
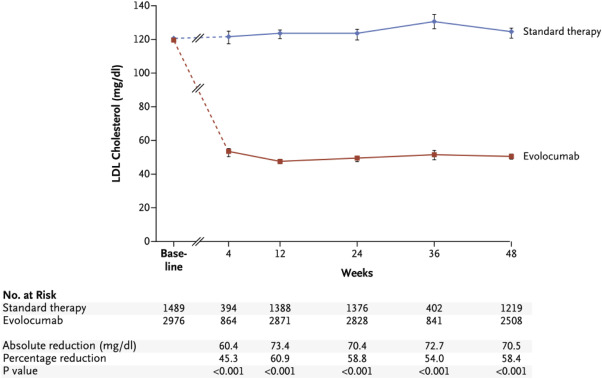
Evolocumab has also been evaluated for the reduction of LDL-C levels in large, randomized clinical trials. The Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER)-1 and OSLER-2 trials were randomized, open-label extension studies that examined longer term outcomes in patients who participated in one of 12 phase 2 (OSLER-1) or phase 3 (OSLER-2) clinical trials of evolocumab. The phase 2 and phase 3 studies enrolled a variety of patient populations, including patients with HeFH, statin intolerance, high levels of cardiovascular risk, CAD, and high levels of persistent LDL-C despite statin treatment. Patients were randomly assigned to treatment with evolocumab (140 mg every 2 weeks or 420 mg monthly) plus standard therapy or standard therapy alone. Long-term outcomes from OSLER-1 and OSLER-2 were recently reported, with a median follow-up of 11.1 months. Compared with standard therapy, evolocumab significantly reduced mean LDL-C by 61% (from a median of 120 mg/dl at baseline to 48 mg/dl at end point; p <0.001; Figure 3 ). After 1 year of open-label treatment, cardiovascular events were noted for 2.18% of patients with standard therapy versus 0.95% with evolocumab (relative risk reduction 53%; p = 0.003).
The recent Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL-C in Subjects Currently not Receiving Drug Therapy for Easing Lipid Levels-2 randomized, controlled clinical trial compared both biweekly and monthly evolocumab versus placebo and oral ezetimibe in patients with a baseline LDL-C of ≥100 and ≤190 mg/dl and a Framingham score of ≤10%. With biweekly administration, mean LDL-C concentration at 12 weeks increased by 0.1% in placebo-treated patients versus a decrease of 17.8% with ezetimibe and 57.0% with evolocumab 140 mg every other week (p <0.001 vs placebo and ezetimibe groups). With monthly treatment, the mean decrease in LDL-C concentration between baseline and 12 weeks was 1.3% with placebo, 18.6% with ezetimibe, and 56.1% with evolocumab 420 mg (p <0.001 vs placebo and ezetimibe groups). The ongoing Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 3 study is a phase 3, multicenter, randomized, double-blind, ezetimibe-controlled clinical trial that is comparing the efficacy of 24 weeks of evolocumab (420 mg monthly) versus ezetimibe (10 mg daily) in patients with hypercholesterolemia who are unable to tolerate an effective statin dose. The 2 coprimary efficacy end points are the mean percent change from baseline in LDL-C at weeks 22 and 24, and percent change from baseline in LDL-C at week 24.
Finally, the 2-part Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities (TESLA) study examined the efficacy and safety of evolocumab in patients with HoFH. TESLA Part A was a phase 2 study that enrolled 8 patients with HoFH who were on stable drug therapy, including 6 patients with receptor-defective HoFH and 2 patients with receptor-negative HoFH. All patients were treated with evolocumab 420 mg by SC injection once monthly for a minimum of 12 weeks, followed by evolocumab 145 mg SC every 2 weeks for another 12 weeks. In the 6 patients with receptor-defective disease, evolocumab decreased mean LDL-C concentration by 19.3% with 4-week dosing and by 26.3% with 2-week dosing, respectively (p <0.0313 vs baseline for each dosing regimen). No reduction in LDL-C concentration was observed in the 2 patients with receptor-negative HoFH. TESLA Part B was a phase 3 study that enrolled 49 patients with HoFH and an LDL-C of ≥130 mg/dl who were on stable doses of statin therapy and other lipid-lowering medications. Patients were randomized to double-blind evolocumab 420 mg or placebo every 4 weeks for 12 weeks. Compared with placebo, patients with HoFH who were randomized to evolocumab exhibited a mean reduction in LDL-C concentration of 30.9% at 12 weeks. Although it is often assumed that patients with HoFH have no LDL-R expression capacity, this is rarely the case. As shown in the TESLA studies, most patients have some capacity to express LDL-R, and inhibition of PCSK9 activity is an effective treatment option in these patients.
PCSK9 monoclonal antibody meta-analysis
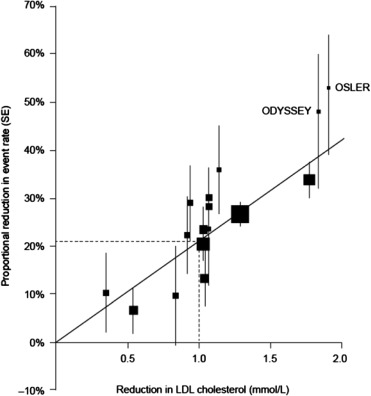
The effects of anti-PCSK9 monoclonal antibodies on cholesterol levels and cardiovascular outcomes were examined in a recent meta-analysis of phase 2 and phase 3 clinical trials. The investigators included 24 randomized controlled trials that compared anti-PCSK9 antibodies versus no anti-PCSK9 therapy in adults with hypercholesterolemia. Patients in the no anti-PCSK9 group received either placebo or ezetimibe. Together, the studies had a combined population of 10,159 patients who were followed up for a mean of 44.6 weeks. For the overall study population, patients who were randomized to treatment with anti-PCSK9 monoclonal antibodies exhibited a significant lowering of LDL-C levels from baseline, with a mean posttreatment difference in LDL-C reduction between treated and untreated groups of 47.49% (p <0.0010). In addition, anti-PCSK9 therapy was associated with significant reductions in the rates of myocardial infarction (MI; odds ratio, 0.49; p = 0.03) and all-cause mortality (odds ratio, 0.45; p = 0.015). As noted previously, a meta-analysis of 14 lipid-lowering studies conducted by the Cholesterol Treatment Trialists’ Collaboration demonstrated that each reduction in LDL-C concentration of 1.0 mmol/L produced a reduction in CVD events of approximately 20%. However, as shown in Figure 4 , the potential benefit of PCSK9 inhibition may be even greater than that of the statins, as CVD event reductions in clinical trials of alirocumab and evolocumab were higher than would be expected for patients achieving similar reductions of LDL-C on statin therapy ( Figure 4 ).
Safety and tolerability
PCSK9 inhibitors are generally well tolerated. In clinical trials of alirocumab, the most commonly occurring adverse events (≥5% of patients treated with alirocumab and more frequently than placebo) were nasopharyngitis, injection-site reactions, and influenza. Liver enzyme abnormalities were reported in 2.5% of patients treated with alirocumab versus 1.8% of placebo-treated patients. In clinical trials of evolocumab, the most common adverse events included nasopharyngitis, upper respiratory tract infection, influenza, back pain, and injection-site reactions.
Longer term outcome trials are continuing to study cardiovascular risk reduction with PCSK9 therapy, as well as the safety and tolerability of the low posttreatment LDL-C concentrations that are possible with these agents. These include the ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment with Alirocumab (ODYSSEY Outcomes) study, which is examining the effects of alirocumab in 18,000 patients with recent acute coronary syndromes, and the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) study, which is examining evolocumab in 27,500 patients with a history of MI, stroke, or peripheral artery disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


