Percutaneous epicardial access (EpiAcc) is used in an attempt to improve outcomes of ablation. We aim to report our experience in EpiAcc for management of symptomatic ventricular premature complexes (VPC) and ventricular tachycardia (VT). All patients from January 2004 to July 2014 who underwent EpiAcc as part of a VPC or VT ablation procedure were included. Outcomes between those with endocardial-only (Gp1) and endocardial/epicardial (Gp2) ablation and those for VPC and VT ablation were compared. EpiAcc for VPC or VT ablation was attempted in 173 patients; 10 patients were excluded because of failure of access (n = 7) or no ablation performed (n = 3). Of the remaining 163, 131 patients (80.4%) had undergone previous endocardial ablation. Mean age was 53.7 ± 15.7 years; 115 (71%) were men. VT ablation was the indication in 105 patients (64%). The underlying substrate was predominately nonischemic cardiomyopathy (49.1%). Epicardial ablation was performed in 115 (70.6%). Procedural and clinical success was obtained in 92.0% and 69.9% of patients, respectively, with no difference between Gp1 and Gp2. Those who underwent VPC ablation had superior clinical outcomes at 1-year follow-up. EpiAcc is feasible in almost all patients with no previous cardiac surgery and permits acute procedural success in >90% of patients, most of whom had failed previous ablation. However, epicardial ablation was not delivered in 1/3 of patients. Epicardial mapping may be helpful as in the absence of an appropriate epicardial site for ablation, and focus can be shifted to more detailed endocardial mapping and ablation.
Here, we determine the rate of epicardial ablation in our institution and compare outcomes between those who underwent endocardial-ablation alone versus endocardial/epicardial ablation. Furthermore, we report outcomes in patients who underwent EpiAcc for the symptomatic ventricular premature complexes (VPC) versus ventricular tachycardia (VT).
Methods
Following institutional review board approval, we enrolled patients who underwent EpiAcc as part of a VPC or VT (nonsustained and sustained) ablation procedure at Mayo Clinic, Rochester, Minnesota, from January 01, 2004, to July 31, 2014. Patients who underwent EpiAcc for supraventricular arrhythmia ablation, atrial appendage closure, or those who had no endocardial/epicardial ablation despite EpiAcc were excluded. Data were abstracted from the record. Patients were grouped according to whether they had isolated endocardial ablation (group 1) versus epicardial ablation either alone or in association with endocardial ablation (group 2).
The decision for epicardial access (EpiAcc) was based on failure of previous endocardial ablation, suggestion of an epicardial exit from the electrocardiogram, or intraprocedural findings. EpiAcc was usually obtained at the start of the procedure and performed under moderate sedation or general anesthesia. Anticoagulation was typically reversed at the time of epicardial puncture if the decision to obtain EpiAcc was made after systemic heparinization, at the discretion of the operator. For anterior puncture, 15° to 30° elevation off the skin surface was used during access, whereas for inferior access the needle was directed at 45°. Once guidewire position was confirmed in right anterior oblique and left anterior oblique views, introducer and sheath were advanced over it. Standard mapping techniques were used. If VT was tolerated, activation and entrainment mapping were used. When the VT was not tolerated or unable to be sustained, substrate and pace mapping were performed. Three-dimensional electroanatomical maps (CARTO; Biosense Webster, Inc., Diamond Bar, California) were created using a 3.5-mm open-irrigated tip catheter. Intracardiac signals were filtered at 30 to 500 Hz. For VT ablation, programmed stimulation was performed with up to 3 ventricular extrastimuli and delivered from ≥2 different sites in all patients. Intravenous isoproterenol was used as needed. For the purposes of this study, a circuit was deemed to have epicardial involvement when epicardial ablation was delivered. Radiofrequency energy was typically applied for 60 to 120 seconds aiming for 10 Ω impedance decrease or guided by temperature; power output started at 30 W and increased to 45 W. The temperature limit was set at 41°C. In regions proximate to phrenic nerve or coronary arteries, high-output pacing and coronary angiography were performed, respectively. Intracardiac echocardiography was used as standard. Epicardial pigtail catheters were left in at the end of the procedure per operator discretion. Since 2008, intrapericardial corticosteroids were administered at the operator’s discretion. If a pigtail catheter remained in situ, transthoracic echocardiography before removal of the catheter was performed once drainage was <50 ml in a 24-hour period. Follow-up was determined from chart review. Arrhythmia recurrence was identified by history, clinical symptoms, noninvasive monitoring devices and, where possible, device interrogation. An “event” was defined as occurrence of any of the following: loss of access, pericardial effusion (greater than small on echocardiography ), chronic pericarditis (≥2 weeks), phrenic nerve injury, pleural injury, coronary vessel injury, abdominal injury (abdomino-pericardial fistula, abdominal wall hematoma, and liver damage), and blood transfusion within 1 week of procedure. A “complication” is an event causing permanent harm or disability or requiring a separate procedure and includes cardiac tamponade (requiring repeat EpiAcc). “Procedural” success was defined as elimination/termination and noninducibility of clinical VPC/VT; nonclinical VPC or VTs were disregarded. The procedure was a failure if we were unable to eliminate the clinical VPC or VT. “Clinical success” was defined as absence of symptoms or arrhythmia (on or off antiarrhythmic therapy); failure was based on symptoms or arrhythmia recurrence despite antiarrhythmic therapy or the need for repeat ablation.
Categorical variables were compared between groups using the chi-square test for independence. Factors that were rare were compared using Fisher’s exact test. Continuous variables were compared using Wilcoxon rank-sum tests. The overall rate of success and survival after the ablation was estimated using the Kaplan-Meier method. Risk factors for success were evaluated using Cox proportional hazards models.
Results
One hundred seventy-three patients underwent attempted EpiAcc as part of a VPC or VT ablation; 3 did not undergo ablation (no ablation target [n = 2], procedural complication before ablation [n = 1]) and 7 failed EpiAcc precluding epicardial mapping and were excluded. Of the remaining 163 patients, 115 (71%) were men. The mean age was 53.7 ± 15.7 years. The average ejection fraction (EF) was 45 ± 16%. VT was the indication in 105 patients (64%). The underlying substrate was predominately nonischemic cardiomyopathy (NIC, “idiopathic”). Sixteen patients had undergone previous sternotomy. Of the overall cohort, 131 patients (80%) had undergone previous endocardial ablation (median 1, range 0 to 5; median time from last procedure 0.5 years, range 1 day to 19 years). Only 6 patients (4%) had undergone previous EpiAcc for ablation. Patients failed an average of 1.7 ± 1.2 antiarrhythmic drugs. Those with combined endocardial/epicardial ablation were more likely to have VT as the procedural indication ( Table 1 ). Otherwise, there were no clinical differences at baseline between groups including presence of defibrillators (57% vs 50% in those with and without epicardial ablation, respectively [p = 0.39]).
| Variable | Epicardial ablation | P-value | |
|---|---|---|---|
| No (N=48) | Yes (N=115) | ||
| Age (years, mean ± SD [median]) | 52.3±17.6 (54.5) | 52.8±14.9 (53.0) | 0.80 |
| Male | 35(72.9%) | 80(69.6%) | 0.67 |
| BMI (Kg/m 2 , mean ± SD [median]) | 30.0±5.6 (29.7) | 29.2±6.4 (28.9) | 0.24 |
| Creatinine (mg/dL, mean ± SD [median]) | 1.3±2.1 (1.0) | 1.1±0.7 (1.0) | 0.88 |
| Hemoglobin (g/dL, mean ± SD [median]) | 13.5±2.4 (13.8) | 13.7±1.6 (15.4) | 0.94 |
| Ejection fraction (%, mean ± SD [median]) | 44.9±15.7 (47.5) | 44.4±15.4 (45.0) | 0.96 |
| Indication | 0.03 | ||
| VPC | 23(52%) | 35(30%) | |
| VT | 25(48%) | 80(70%) | |
| Substrate | |||
| NIC | 24(50%) | 56(49%) | 0.88 |
| IC | 5(10%) | 16(14%) | 0.54 |
| TIC ∗ | 3(6%) | 4(4%) | 0.42 ‡ |
| RV abnormality † | 2(4%) | 16(14%) | 0.10 ‡ |
| Sarcoid | 1(2%) | 0 | 0.29 ‡ |
| Normal | 12(25%) | 21(18%) | 0.33 |
| Other | 1(2%) | 2(2%) | 1.0 ‡ |
| # of previous endocardial ablations, mean ± SD (median) | 1.4±1.1 (1) | 1.4±1.1 (1) | 0.92 |
| Prior epicardial access for ablation | 1(2%) | 5 (4%) | 0.67 ‡ |
| Hypertension | 23(48%) | 59(51%) | 0.69 |
| Diabetes mellitus | 7(15%) | 16(14%) | 0.91 |
| Renal disease | 7(15%) | 16(14%) | 0.79 |
| Coronary artery disease | 10(21%) | 18(16%) | 0.42 |
| Obstructive sleep apnea | 13(28%) | 23(20%) | 0.30 |
| Chronic obstructive lung disease | 1(2%) | 11(10%) | 0.11 ‡ |
| Prior cardiac surgery | 6(13%) | 6(5%) | 0.10 |
| CABG | 2(4%) | 2(2%) | 0.36 |
| # of AADs failed, mean ± SD (median) | 1.6±1.2 (1.0) | 1.7±1.2 (2.0) | 0.37 |
∗ Includes PVC-induced cardiomyopathy.
† Includes arrhythmogenic right ventricular cardiomyopathy (ARVC).
Those who had VT (vs VPC) ablation were older (55.5 vs 47.4 years, p <0.001), were more often male (79% vs 55%, p = 0.001), had more cardiac surgery (11% vs 0%, p = 0.005), and lower EF (40% vs 53%, p <0.001). Furthermore, they had a higher incidence of coronary artery disease (23% vs 7%, p = 0.01), hypertension (58% vs 36%, p = 0.008) and renal disease (19% vs 3%, p = 0.005). VT ablation patients had more IC (16% vs 5%, p = 0.04) and fewer had structurally normal hearts (14% vs 31%, p = 0.01). The VT ablation group failed more antiarrhythmic drugs (2% vs 1.2%, p <0.001) before ablation.
Anterior access, inferior access, or both was obtained in 86 (54%), 69 (44%), and 2 patients (1%), respectively. One underwent a hybrid approach with subxiphoid surgical window. Forty-eight patients underwent endocardial-only ablation (group 1); 115 (71%) underwent epicardial ablation (group 2) either alone (14 patients) or combined with endocardial ablation (101 patients). Reasons for endocardial-only ablation were as follows: in 3 patients (2%), adhesions precluded access to site of interest; in 5 (3%), optimal site was proximate to coronary artery or phrenic nerve; in 39 (24%), there was no evidence of epicardial involvement. The reason could not be determined in 1 patient.
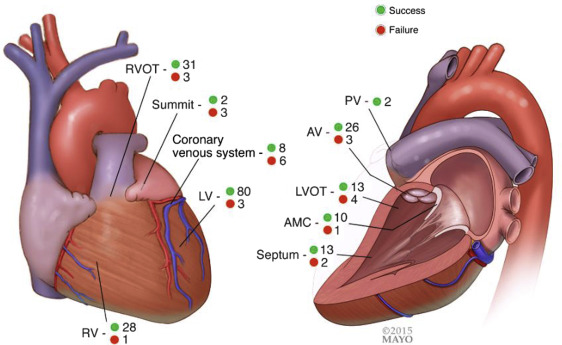
The number of VPC/VTs found and ablated is listed in Table 2 . Ablation site is shown in Figure 1 . Sixty-one patients underwent endocardial and/or epicardial ablation within the outflow tract/aortic valve/pulmonary valve region. Fourteen patients (9%) with EpiAcc underwent ablation through the coronary venous system which we considered endocardial (5 in coronary sinus [CS], 6 in anterior interventricular vein, 2 at coronary sinus-anterior interventricular vein junction, and 1 in posterolateral vein). Of these 14, 3 (2%) underwent ablation through the coronary venous system only despite EpiAcc as sites here were more desirable (n = 2) or could not be reached through EpiAcc because of adhesions (n = 1). Concordant mapping data (comparably early sites both found in the same region) with epicardial and venous-based mapping were obtained in 8 of 14 patients (57%).
| Variable | Epicardial ablation | P-value | |
|---|---|---|---|
| No (N=48) | Yes (N=115) | ||
| Access route (N=158) | 0.22 | ||
| Anterior | 22(48%) | 64(57%) | |
| Inferior | 23(50%) | 46(41%) | |
| Both | 0 | 2(2%) | |
| Surgical (hybrid) | 1(2%) | 0 | |
| Type of anesthesia | 0.51 | ||
| Sedation | 27(56%) | 68(61%) | |
| General anesthesia | 21(44%) | 44(38%) | |
| # of VTs found (VT group only [N=104], mean ± SD [median]) | 3.2±1.9 (3.0) | 3.4±2.6 (3.0) | 0.79 |
| # of VPCs found (VPC group only [N=56], mean ± SD [median]) | 1.2±0.5 (1.0) | 1.6±0.8 (1.0) | 0.02 |
| Clinical VTs ablated (VT group only [N=104]), mean ± SD [median]) | 1.7±1.1 (1.0) | 1.8±1.1 (1.0) | 0.58 |
| Clinical VPCs ablated (VPC group only [N=56], mean ± SD [median]) | 1.2±0.5 (1.0) | 1.4±0.5 (1.0) | 0.08 |
| Study time (mins, mean ± SD [median]) | 371.4±125.2 (383.0) | 413.9±125.2 (395.5) | 0.12 |
| Fluoroscopy time (mins, mean ± SD [median]) | 76.8±31.0 (67.0) | 81.1±35.0 (73.0) | 0.69 |
| Total rads (mean ± SD [median]) | 2403.7±1508.6 (2094.5) | 2014.9±1380.3 (1839.5) | 0.13 |
| Intrapericardial steroids | 16(33%) | 44(38%) | 0.55 |
| Intrapericardial lidocaine | 15(34%) | 52(45%) | 0.19 |
| Heparin | 45(96%) | 114(99%) | 0.15 |
| Aspirin | 24(50%) | 65(57%) | 0.45 |
| Clopidogrel | 1(2%) | 5(4%) | 0.67 ∗ |
| Warfarin | 28(58%) | 59(52%) | 0.44 |
| Average ACT (mean ± SD [median]) | 258.0±56.0 (265.2) | 240.4±61.6(239.4) | 0.07 |
Mean fluoroscopy and procedural times were 79.9 ± 33.8 and 401.3 ± 126.3 minutes, respectively. A pigtail pericardial catheter was left in situ in 35 patients (21%; mean duration <1 day). Intrapericardial steroids and lidocaine were administered in 60 (37%) and 68 (40%) patients, respectively.
Compared with the VPC ablation group, the VT ablation group was more likely to receive general anesthesia (49% vs 24%, p = 0.002), had more morphologies found (3.3 vs 1.4, p <0.0001), and ablated (1.8 vs 1.3, p = 0.006). The VT group tended to have epicardial ablation more frequently, but this was not significant (76% vs 60%, p = 0.09). Fluoroscopy and study time were higher in the VT group (85 vs 70 minutes and 422 vs 364 minutes, respectively, p <0.006 for both). There was no difference in complication rates, although the VT group required more blood transfusion (13% vs 2% of patients, p = 0.02).
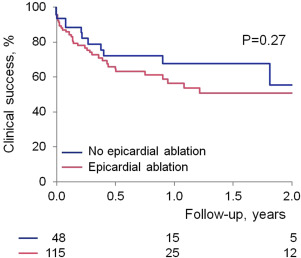
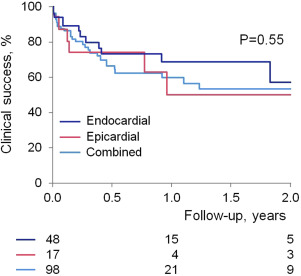
Outcome data were available in all patients. Procedural success was obtained in 150 patients (93% in group 1 vs 90% in group 2, p = 0.41) with no difference between those with or without structural heart disease nor between VPC versus VT indication. Procedures in which coronary venous system ablation was delivered and those who had ablation in the summit region had lower procedural success rate compared with those without ablation in these regions (57% vs 95%, p <0.001, and 40% vs 94%, p = 0.004). Clinical success was maintained, at an average of 1 year follow-up, in 114 patients with no difference between group 1 and 2 or between substrates ( Figures 2 and 3 ). At last follow-up, 83 patients (51%) were on antiarrhythmic drugs (average 1.2). Median recurrence time was 67 days. Of note, 21 patients had subsequent ablations (5 in group 1 and 16 in group 2, p >0.05); the time to subsequent ablation was not different.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


