Chapter 58 Nonischemic Dilated Cardiomyopathy
Diagnosis and Management
Introduction and Classification
Nonischemic dilated cardiomyopathy (DCM) is the most common form of cardiomyopathy. The hallmarks of DCM are left or often biventricular enlargement with mostly global systolic hypokinesis, although some regionally more pronounced contraction abnormality may be present.1 Several specific diseases of the heart muscle (e.g., infectious agents, chemotherapeutic agents, metabolic disorders, genetic mutations) present as clinical manifestations of DCM, which presumably represents a final common pathway of myocardial damage.1 Therefore, DCM is defined as a primary cardiomyopathy with a mixed etiologic background. Nevertheless, a considerable overlap between the different groups must be taken into account (e.g., myocarditis and DCM caused by an infectious agent). The definitions of DCM and ischemic cardiomyopathy, with the latter defined as a “dilated cardiomyopathy with impaired contractile performance not explained by the extent of the coronary artery disease or ischemic damage,” have been controversial in the past.2 The recent definitions and the classification of cardiomyopathies by the American Heart Association do not include pathologic myocardial processes and dysfunction caused directly by other cardiovascular diseases; hence the term ischemic cardiomyopathy is not supported any more.1 Therefore, although this term continues to be used, it is not directly linked to the previous definition but is meant to reflect any type of left ventricular dysfunction caused by coronary artery disease.2 In this context, one should concede that without coronary angiography, the diagnosis of significant coronary artery disease solely based on clinical findings in patients with heart failure often fails to identify this cause.3
Epidemiology and Survival
The incidence of DCM varies from 5 to 8 cases per 100,000 per year, and the prevalence is estimated to be 1 : 2500, with approximately 10,000 deaths and 46,000 hospitalizations in the United States.1 Heart failure caused by DCM represents a major health issue and is the primary indication for heart transplantation.4 Usually, DCM presents for the first time in patients between 18 and 50 years of age; however, children and older adults may also be affected.5 Furthermore, it develops almost three times more often in blacks and males than in whites and females, with apparently lower survival rates in blacks than in whites for unknown reasons.
Mortality and Causes of Death in Dilated Cardiomyopathy
The natural history of DCM is diverse. Some patients have minimal or no symptoms, whereas symptomatic patients usually experience a progressive deterioration; however, a minority improves with a reduction in cardiac size and longer survival. In some patients, clinical and functional improvements may occur years after the initial manifestation of symptoms. Recently, better survival rates have been achieved with improvement in medical (angiotensin-converting enzyme [ACE] inhibitors, β-blockers, aldosterone inhibitors) and device therapy (implantable cardioverter-defibrillator [ICD] and cardiac resynchronization therapy).6 A difference between nonfamilial and familial cases of DCM could not be demonstrated.7 Yet, the prognosis of some secondary cardiomyopathies, for example, the human immunodeficiency virus (HIV)–related form, is particularly bad. Irrespective of the underlying cause, patients with DCM are prone to ventricular arrhythmias and sudden cardiac death (SCD), with DCM representing the substrate for approximately 10% of all SCDs in adults.8 Approximately 20% of patients with DCM will die within 1 year after diagnosis; in most of them, the cause is SCD.9
Genetics and Other Causes of Dilated Cardiomyopathy
DCM presumably represents a final common or toxic pathway that is the end result of myocardial damage caused by different mechanisms.1 In about 50% of cases, patients are described as having “idiopathic” DCM because an etiologic cause or secondary reason cannot be identified.10 Several specific diseases of the heart muscle or metabolism can lead to the clinical manifestations of DCM. Evidence that many idiopathic cases still result from inherited abnormalities is increasing, since 20% to 50% of these may be familial on further evaluation.11 Genetically determined familial DCM, which refers to the presence of two or more family members with DCM, can be subdivided into at least four phenotypes12: isolated DCM, DCM with involvement of the cardiac conduction system, DCM with concomitant skeletal myopathy (with or without conduction disease), and DCM with sensorineural deafness.13 The most common mode of inheritance is autosomal dominant (56%), and in 5% to 10%, it is linked to the X chromosome.14–16 Autosomal recessive or mitochondrial forms of DCM are uncommon.17–19 Molecular analysis has revealed a great number of genes and chromosomal loci leading to DCM. The pathophysiological effects are a malfunction of force generation (because of mutations in sarcomeric protein genes) and of force transmission (due to mutations in cytoskeletal protein genes).20,21 Other causes of DCM as a secondary cardiomyopathy may be infectious disease, a tachycardiomyopathy, inflammatory cardiomyopathy, deposition diseases, medications, toxins, endocrinologic disorders, neuromuscular diseases, rheumatologic diseases, postpartum cardiomyopathy, uremia, and others.1 These causes have to be taken into account whenever a genetic cause is considered.
Pathophysiology of Arrhythmias in Dilated Cardiomyopathy
Multiple mechanisms contribute to the development of ventricular arrhythmias in patients with dilated cardiomyopathy.22 Autopsy studies have shown substantial left ventricular subendocardial scarring in 33% of patients and patchy areas of replacement fibrosis in 57%, accompanied by increased perivascular fibrous tissue and perimyocytic fibrosis in the left ventricle.23 This may be the substrate for re-entry. Other factors such as hypokalemia, hypomagnesemia, and ischemia caused by the occlusion of small intramyocardial arteries by thrombosis or emboli may serve as triggers for ventricular arrhythmias.24 An elevated sympathetic tone and increased circulating catecholamines may also favor ventricular re-entrant arrhythmias.25,26 Stretch-induced shortening of the ventricular refractory period may support the development of re-entry.27
A distinct form of ventricular arrhythmia in DCM is bundle branch re-entrant ventricular tachycardia (BBRVT). A macro–re-entry, which usually employs the right bundle branch as the antegrade limb and the left bundle branch as the retrograde limb, leads to a rapid ventricular tachycardia (VT). Apart from BBRVT, macro–re-entrant VTs involving myocardial scars at the mitral annulus are frequently observed.28,29
Ventricular arrhythmias in patients with chronic heart failure caused by DCM may be provoked by non–re-entrant mechanisms such as abnormal automaticity and triggered activity.30,31 The occurrence of triggered activity and the causative early after-depolarizations is promoted by prolonged repolarization and prolonged action potential predominately induced by the downregulation of repolarizing potassium channels.32,33 Focal ventricular arrhythmias originating from the distal Purkinje system are often nonsustained.30,31 Although frequently occurring in patients with DCM, VT is not the only cause of SCD in these patients. At end-stage heart failure, bradycardia and electromechanical dissociation are very common causes of SCD.34
Risk Stratification for Arrhythmic Death in Dilated Cardiomyopathy
Left Ventricular Function
In patients with nonischemic DCM, overall mortality is associated with left ventricular dysfunction, but only a few studies have investigated the relationship between left ventricular function and SCD directly.35 The combination of severely reduced left ventricular function (left ventricular ejection fraction [LVEF] <30%) and nonsustained VT was used to identify the highest-risk subgroup.36 Since reduced LVEF was an inclusion criterion in all ICD prophylaxis trials, it is a prominent feature of guidelines for ICD therapy and a cornerstone in daily clinical practice.37,38 Nevertheless, the majority of SCDs occur in patients with less severely reduced left ventricular function, which highlights the limited sensitivity of this parameter.39
Electrocardiography
In patients with nonischemic DCM, the presence of left bundle branch block (LBBB) has been associated with a worse outcome. The Vesnarinone Trial (VEST) and other studies confirmed a significant association between the degree of QRS duration and mortality.40–42 However, other studies were not able to demonstrate a significant association between intraventricular conduction delay and SCD.36,43,44 The Defibrillators in Nonischemic Cardiomyopathy (DEFINITE) trial was not able to show an association between QRS duration and all-cause mortality.45 The Sudden Cardiac Death–Heart Failure Trial (SCD-HeFT), which enrolled patients with ischemic cardiomyopathy and those with nonischemic cardiomyopathy, reported that ICD therapy yielded a greater mortality reduction in patients with QRS duration ≥0.12 seconds, but specific information on the relationship between QRS duration and mortality reduction in patients with nonischemic cardiomyopathy has not been presented.46 In a retrospective analysis of the Congestive Heart Failure Survival Trial of Antiarrhythmic Therapy (CHF-STAT) database, Iuliano et al identified a prolonged QRS duration of ≥0.12 seconds as an independent predictor of total mortality and SCD in patients with heart failure.47 The role of atrial fibrillation remains controversial, too. Survival rates may be reduced by increased thromboembolic events or ventricular arrhythmias, which may be evoked by an increased dispersion of refractoriness caused by long-short cycle lengths.48
Spontaneous Ventricular Arrhythmias
The prevalence of spontaneous ventricular arrhythmias in patients with DCM is very high.49 Polymorphic premature ventricular contractions, ventricular pairs, and nonsustained VT are very common, with increasing prevalence of nonsustained VT and increasing severity of heart failure symptoms. The positive predictive value of these arrhythmias is relatively low, ranging from 20% to 50%, yet the negative predictive value has been cited as high as 95.5%.50,51 In the absence of a treatment modality with proven efficacy, specific treatment for nonsustained VT is not indicated, except in the rare circumstances of symptomatic, frequent, or very rapid episodes leading to hemodynamic instability.
Heart Rate Variability, Baroreflex Sensitivity, and Heart Rate Turbulence
Heart rate variability (HRV) and baroreflex sensitivity (BRS) provide indirect (i.e., through their effects on the sinus node) measures for the autonomic effects in the ventricle that may be important in the pathophysiology of VT and SCD. Most studies using HRV, BRS, and heart rate turbulence (HRT) as predictors of adverse arrhythmic events have been conducted in patients after the occurrence of myocardial infarction (MI). One of the few studies in DCM was conducted by Rashba et al.52 They reported a significant difference in mortality rates in a substudy of the Defibrillators in Nonischemic Cardiomyopathy (DEFINITE) trial. With decreasing standard deviation of normal R-R intervals (SDNN) as a measurement of HRV, an increase in total mortality was noted: Among 70 patients with SDNN longer than 113 ms, no deaths occurred. However, in 69 patients with SDNN between 81 and 113 ms, the mortality rates was 7%; in 72 patients with SDNN less than 81 ms, the mortality rate was 10%. Similar results were seen when the predictive value of SDNN was examined for the composite endpoint of SCD and appropriate ICD shock. On the basis of this study, the authors suggested that patients with nonischemic DCM and preserved HRV have a good prognosis and may not benefit from ICD prophylaxis and that lower levels of SDNN were associated with a progressively increased mortality risk.
HRT describes the short-term fluctuation in sinus cycle length that follows a ventricular premature beat.53,54 It has been postulated that it measures vagal responsiveness similar to BRS. It is a potentially attractive risk factor, as it can be performed with a relative small number of premature beats from 24-hour Holter electrocardiogram (ECG). In the Marburg Cardiomyopathy Study (MACAS), low HRT was a multivariate predictor of transplant-free survival, but not of arrhythmic events.55 In the same study, blunted BRS, which identified patients with a higher cardiac mortality after a recent MI in the Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) study, was not a predictor of arrhythmic events.56
Microvolt T-Wave Alternans
The term microvolt T wave alternans (MTWA) refers to the presence of beat-to-beat changes in T-wave amplitude that are not detectable on the surface ECG. Bloomfield et al showed that in patients with left ventricular dysfunction (EF ≤40%), the negative predictive accuracy of MTWA was very high (≥98% at a follow-up of 2 to 3 years) regardless of the etiology of the cardiomyopathy, with significantly lower event rates in patients with normal MTWA test results.57 In contrast, a prospective substudy of the SCD-HeFT trial, which tested the predictive value of MTWA in 490 (n = 250 nonischemic heart failure) of the 2521 patients with an EF 35% or less and New York Heart Association (NYHA) class II or III heart failure randomized to ICD therapy, amiodarone, or placebo, did not show any significant differences in the composite primary end point of the first occurrence of any of the following: SCD, sustained VT or ventricular fibrillation (VF), or appropriate ICD discharge.58 In conclusion, further evidence is needed in the specific setting of nonischemic DCM.
Cardiac Magnetic Resonance Imaging
Wu et al recently observed that late gadolinium enhancement detected by cardiac magnetic resonance imaging (MRI) strongly predicts adverse cardiac outcomes, including adverse arrhythmic events in patients with nonischemic DCM, supporting earlier reports by Assomull et al.59,60 In the latter study, midwall fibrosis was detected in 35% of patients with nonischemic DCM by late gadolinium enhancement. This finding was associated with a higher rate of the predefined combined primary endpoint of all-cause death and hospitalization for a cardiovascular event and also predicted secondary outcome measures of SCD or VT.
Electrophysiological Testing
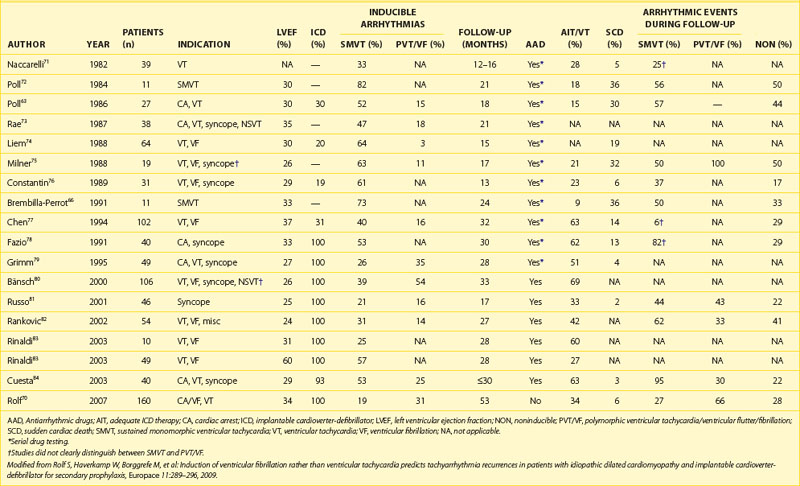
The role of electrophysiological testing (i.e., programmed ventricular stimulation) in risk stratification in patients with nonischemic cardiomyopathy and no history of sustained ventricular arrhythmias has been addressed in nine studies.61–69 The small numbers of patients in each study, the low rate of arrhythmia induction and reproducibility, and the low subsequent arrhythmia event rates have made it difficult to draw consistent conclusions. Therefore, programmed ventricular stimulation is not recommended for risk stratification in patients with nonischemic cardiomyopathy. However, Rolf et al showed that in a relatively large cohort of 160 patients with DCM who received ICDs for secondary prophylaxis, the induction of polymorphic VT or VF in contrast to the induction of monomorphic VT was associated with a high risk of subsequent fast ventricular arrhythmias during a mean follow-up of 53 months (Table 58-1). Nevertheless, a subgroup of patients with secondary prevention and sufficiently low risk that would render ICD therapy unnecessary could not be identified. A prospective study in a DCM population with primary prevention, possibly in combination with other risk factors, might be helpful in refining the indications for ICD therapy.
Management
Vasodilator Therapy
Treatment with ACE inhibitors improves ventricular function, patient well-being and reduces hospital admissions for worsening heart failure. Moreover, a reduction of total mortality and SCD by ACE inhibitor therapy has been demonstrated. Detailed information on the treatment of acute and chronic heart failure, which is beyond the scope of this chapter, is provided in current guidelines.85,86
Antiadrenergic Therapy
The pathophysiological rationale for antiadrenergic therapy in patients with DCM is to antagonize the heightened sympathetic tone and circulating catecholamines, thereby reducing the various adverse effects of these regulatory mechanisms. Several studies have shown symptomatic improvement in patients with DCM treated with β-blockers. To date, no specific studies have demonstrated the benefit of β-blockers for the prevention of SCD in DCM. However, several studies have shown that the reduction in mortality is similar in patients with ischemic heart failure or nonischemic heart failure. The Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) randomized 3991 patients with an LVEF 40% or less and NYHA class II to IV to metoprolol or placebo in addition to conventional therapy. It showed a reduction in overall mortality by 34% at 21 months (30% in patients without coronary artery disease).87 Of note, a 41% reduction in SCD was observed. Similarly, the CIBIS II study showed in 1327 patients (160 patients with nonischemic DCM) in NYHA class III and IV randomized to bisoprolol, a significant 34% reduction in total mortality at 1.3 years because of a 44% reduction in SCD mortality rates.88 Taking these studies and the results of the Carvedilol Heart Failure Study Group (HFSG) trial into account, all patients with congestive heart failure should receive β-blockers and ACE inhibitors unless contraindicated.89
Antiarrhythmic Drugs
Since patients with DCM are prone to atrial and ventricular arrhythmias, antiarrhythmic drugs may be considered for treatment. However, most antiarrhythmic drugs may exhibit proarrhythmic effects and exacerbate the left ventricular dysfunction; the mortality rate may be increased when some class I or III antiarrhythmic drugs are used. Data from the Cardiac Arrhythmia Suppression Trial (CAST) on patients who have had MIs have been extrapolated to others with reduced left ventricular function and to those with structural heart disease in general.90 Therefore, despite the fact that patients with DCM frequently have symptomatic as well as asymptomatic supraventricular and ventricular arrhythmias, treating them with class Ia and Ic drugs is not advised. This also applies to D-sotalol.91–93 Proarrhythmia rarely occurs with amiodarone therapy, even in patients with depressed left ventricular function. Therefore, amiodarone is the most frequently used antiarrhythmic agent in patients with DCM. Of note, patients with nonischemic cardiomyopathy were under-represented in most studies, except for the relatively small Amiodarone Versus Implantable Cardioverter-Defibrillator: Randomized Trial in Patients with Nonischemic Dilated Cardiomyopathy and Asymptomatic Nonsustained Ventricular Tachycardia (AMIOVIRT) and SCD-HeFT (Sudden Cardiac Death-Heart Failure Trial).46,94 Since no placebo group was included in AMIOVIRT, the role of nonsustained VT for risk stratification in nonischemic DCM and the superior effectiveness of amiodarone compared with placebo remain unclear.
In the CHF-STAT (Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy), amiodarone proved to be more effective in patients with nonischemic cardiomyopathy versus those with ischemic cardiomyopathy with regard to survival without SCD or hospitalization.95 However, a reduction in overall mortality or in mortality from SCD could not be demonstrated in the entire study cohort. The outcomes of patients as a function of NYHA class were not reported.
In contrast to this, in the Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) trial, which was a randomized but open trial, all-cause mortality was reduced by 28% (P < .03), and mortality from SCD was reduced by 27% (P = .056) in the amiodarone treatment group.96 Moreover, a higher percentage of patients improved by NYHA functional class. In comparison to the CHF-STAT, the GESICA trial included fewer patients with ischemic cardiomyopathy (39% vs. 72%) but a significant number of patients with Chagas disease. Hence, the positive results of the CHF-STAT in the case of patients with nonischemic DCM might be confirmed by GESICA. Nevertheless, these two trials present conflicting data on amiodarone treatment for patients with heart failure.
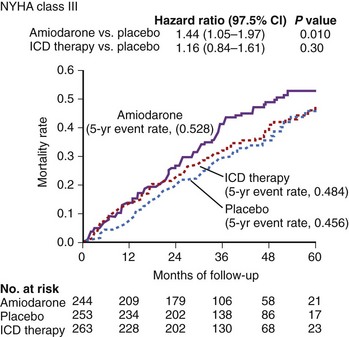
In the larger SCD-HeFT trial, which randomized patients with ischemic or nonischemic cardiomyopathy, an LVEF less than 36%, and a history of congestive heart failure to placebo, amiodarone, or an ICD, no significant difference was seen in all-cause mortality in the placebo group and the amiodarone group, but the ICD significantly reduced overall mortality. Of note, in SCD-HeFT, a relative 44% increase was seen in the risk of death in the amiodarone group compared with the placebo or ICD group among patients with NYHA III heart failure. Thus, although survival in the amiodarone and placebo arms of class II patients was identical, amiodarone was worse than placebo in class III patients (Figure 58-1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree