Nonatherosclerotic Coronary Heart Disease: Introduction
Although atherosclerotic disease of the coronary arteries is the most common cause of luminal narrowing and coronary heart disease, there are multiple nonatherosclerotic (congenital and acquired) causes of severe luminal narrowing and subsequent clinical coronary events (angina pectoris, acute myocardial infarction, and sudden death) (Table 55–1).
|
|
|
| Spasm |
|
|
| Buerger disease |
| Giant cell arteritis |
|
|
|
|
|
|
| Intramural coronary artery disease (small-vessel disease) |
|
| Normal coronary arteries |
Various nonatherosclerotic coronary artery diseases can reduce or interrupt coronary blood flow by three mechanisms: (1) fixed luminal obstructions (internal narrowing), (2) encroachment of the lumen by disease of the arterial wall or adjacent tissues (external narrowing), or (3) both.1 Reduction in coronary arterial blood flow may also result from dynamic changes in the walls of an otherwise normal artery (spasm) or from a disproportion of myocardial oxygen supply and demand. In view of current trends toward rapid coronary artery reperfusion to salvage jeopardized myocardium during evolving acute myocardial infarction, the various nonatherosclerotic etiologies of coronary artery disease must be considered.
Frequency of Nonatherosclerotic Coronary Narrowing Producing Fatal Myocardial Infarction
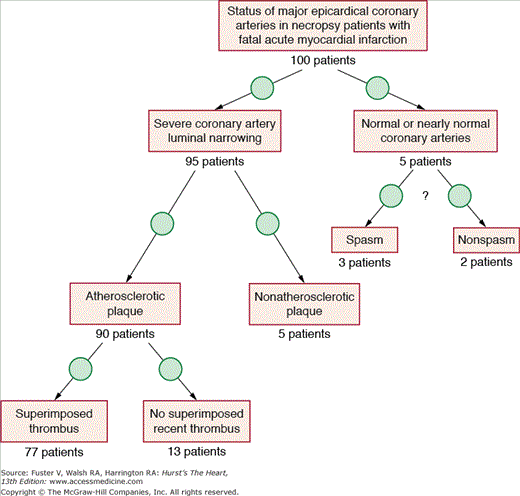
Approximately 4% to 7% of all patients with acute myocardial infarction and nearly four times this percentage of patients younger than age 35 years do not have atherosclerotic coronary artery disease as demonstrated by coronary arteriography, at necropsy, or both.1-6 Because coronary angiography simply represents an image of one lumen, the specificity for etiology of the coronary luminal narrowing is extremely low. Review of necropsy studies1-3 suggests that approximately 95% of patients with fatal acute myocardial infarction have at least one major epicardial coronary artery with severe luminal narrowing or total occlusion (Fig. 55–1). The remaining 5% of patients apparently have normal major epicardial coronary arteries. Of the 95% of patients with severe coronary luminal narrowing, 95% have typical atherosclerotic plaque with a superimposed thrombus in 85%.
Figure 55–1.
Diagram displaying the approximate breakdown of status of major epicardial coronary arteries in necropsy patients with fatal acute myocardial infarction. Reproduced with permission from Waller.1
The remaining 5% of the patients with severe coronary luminal narrowing have a host of etiologies (see Table 55–1), including coronary arteritis, trauma, systemic metabolic disorders, intimal fibrous proliferation, and coronary emboli. In medical centers with large populations of cardiac transplantation patients, the incidence of nonatherosclerotic coronary disease will exceed 5% because of the frequency of intimal fibrous proliferation in the coronary arteries late after transplantation. This is commonly known as transplant arteriopathy. Of the 5% of patients seen at necropsy after fatal acute myocardial infarction with normal or nearly normal epicardial coronary arteries, 50% to 60% likely represent clinical coronary spasm, but the remaining 40% to 50% represent a combination of congenital coronary artery anomalies, spontaneous recanalization, and mismatches of coronary supply and myocardial demand.
In the presence of acute myocardial infarction, there is a >90% chance that the underlying etiology in the Western world is coronary atherosclerosis. Many other diseases have been associated with myocardial infarction.5 The epicardial coronary arteries, intramural coronary arteries, or both are affected (see Table 55–1).6
Congenital Coronary Artery Anomalies
Variation in the origin, course, or distribution of the epicardial coronary arteries is found in 1% to 2% of the population (Table 55–2; Fig. 55–2).1,7-13
| Anomalous origin of one or more coronary arteries from the aorta |
|
|
|
|
| High-takeoff coronary ostia |
|
| Coronary artery fistula |
| Myocardial bridges |
Figure 55–2.
Diagram showing various congenital coronary artery anomalies that are associated with clinical symptomatic heart disease. A, anterior cusp; Ao, aorta; L, left cusp; LAD, left anterior descending; LC, left circumflex; LM, left main; P, posterior cusp; PT, pulmonary trunk; R, right cusp or right coronary artery.
Certain types of these anomalies—including ostial lesions, passage of a major artery between the walls of the pulmonary trunk, a major coronary artery originating from the pulmonary trunk, or perhaps myocardial “bridges”—may produce ischemia with subsequent myocardial infarction.
The incidence of coronary anomalies at routine autopsy varies from 0.3% to 0.6%,12 whereas the incidence of major coronary anomalies, causing acute myocardial infarction at autopsy (<35 years of age), has been reported as 4% (5 in 120). Angiographically, the incidence of coronary arterial anomalies ranges from 0.6% to 1.55%. Virmani and Forman5 reported 232 necropsy patients at the Armed Forces Institute of Pathology with isolated coronary anomalies associated with sudden death (see Table 55–2).
Origin of Both Right and Left Coronary Arteries from the Same Sinus of Valsalva
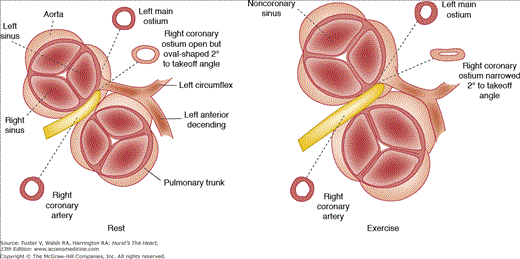
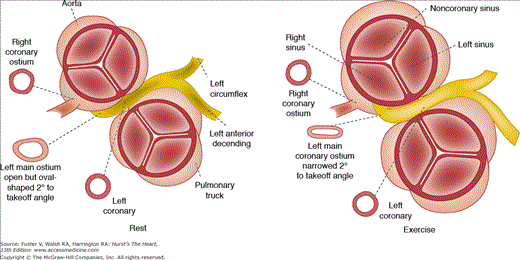
When either the right or left coronary artery arises from the left or right sinus of Valsalva, respectively, the anomalous vessel transverses the base of the heart in a course anterior to the pulmonary trunk, posterior to the aorta, or between the aorta and pulmonary trunk (Figs. 55–3 and 55–4). At least 43 cases have been reported with necropsy where the origin of the left main coronary artery is from the right sinus with passage between the aorta and pulmonary trunk.7 In 79% of these patients, death was related to the anomaly, with sudden death or an acute myocardial infarction. At necropsy, 5 of 26 patients younger than age 20 years had myocardial infarcts.7 When the right coronary artery originates from the left sinus of Valsalva and passes between the aorta and pulmonary trunk, symptoms of myocardial ischemia, infarction, or sudden death may occur.7 Of 12 patients with this anomaly, 3 died suddenly and 2 had angina or syncope. At necropsy, transmural ventricular scars (healed infarction) were seen in two patients.
Figure 55–3.
Diagram showing the proposed mechanism of myocardial ischemia produced by anomalous origin of the right coronary artery from the left sinus of Valsalva. With exercise, the aorta and pulmonary trunk dilate, thereby reducing the already narrowed coronary ostium of the anomalous right coronary. From Roberts WC, et al. Origin of the right coronary artery from the left sinus of valvsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. Mar 1982;49(4):863-868.
Figure 55–4.
Diagram showing the proposed mechanism of myocardial ischemia produced by anomalous origin of the left coronary artery from the right sinus of Valsalva. With exercise, the aorta and pulmonary trunk dilate, thereby reducing the already narrowed coronary ostium of the anomalous left coronary. From Roberts WC, et al. Origin of the right coronary artery from the left sinus of valvsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. Mar 1982;49(4):863-868.
The mechanism of ischemia, infarction, and/or sudden death in this coronary anomaly appears related to the shape of the coronary ostium of the anomalous vessel (see Fig. 55–4). Normally, the coronary ostia are round to oval in shape, but in this anomaly, the coronary artery has an acute angle of takeoff that makes the ostium slit-like in shape. With increased cardiac output, the aorta dilates with stretching of the aortic wall, so that this slit-like ostium may become severely narrowed (Figs. 55–3, 55–4, 55–5, 55–6). It is unlikely that there is “compression” of the anomalous coronary artery by the aorta and pulmonary trunk, in view of the marked differences in diastolic pressures. There is likely an anterior shift of the anomalous vessel rather than a vise-like compression.
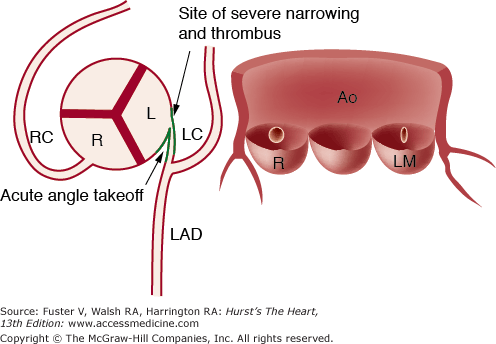
Figure 55–5.
Diagram showing acute-angle takeoff of the left main coronary artery with ostial ridge and slit-like orifice. The proximal left main is occluded by atherosclerotic plaque and thrombus, but the remaining vessels are normal. Accelerated coronary atherosclerosis may result from the acute-angle takeoff malformation. Ao, aorta; L, left cusp; LAD, left anterior descending; LC, left circumflex; LM, left main; R, right cusp; RC, right coronary. Reproduced with permission from Menke DM, Jordan MD, Sut CH, Aust CH, Waller BF. Isolated and severe left main coronary atherosclerosis and thrombosis: a complication of acute angle takeoff of the left main coronary artery. Am Heart J. 1986;112:1319-1320.
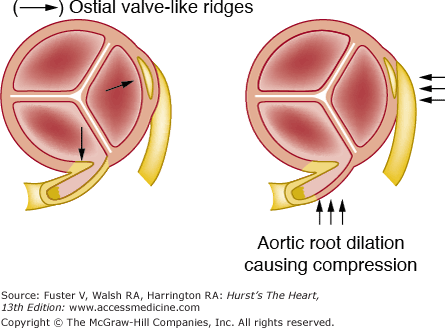
Figure 55–6.
Diagram illustrating ostial valve-like ridges and the proposed mechanism of ostial compression with aortic root dilation. Reproduced with permission from Virmani et al.10
Single Coronary Artery
Origin of the entire coronary circulation from a single aortic ostium is termed single coronary. This anomaly is rare in the absence of other associated anomalies of the heart (see Fig. 55–1). One or more branches of the single artery may cross the base of the heart in a fashion described earlier and thus may be exposed to the risks of ischemia due to acute angulation. Angina pectoris and myocardial lactate production indicating myocardial ischemia have been demonstrated in patients with single coronary arteries where coronary atherosclerosis or an anomalous coronary artery passage was absent.
Coronary Artery Atresia
High-Takeoff Coronary Ostia
Normally, the coronary ostia are located within the sinuses of Valsalva, which optimizes coronary artery blood flow in diastole. Location of the ostia in the tubular portion of the aorta (ie, “high-takeoff” position) may be associated with decreased coronary perfusion (Figs. 55–7 and 55–8). Morphologic evidence of chronic ischemia has been reported in a patient with a high-takeoff right coronary artery who had right and left ventricular wall scarring.11-13 High-takeoff position of the coronary ostium also has been postulated as a cause of sudden coronary death. In a series of 54 major and minor coronary artery anomalies, coronary artery ostia arose above the sinotubular junction in 2, the right coronary artery ostium arose high in 5, and the left coronary artery ostium was in a high-takeoff position in 3.14 In two cases of high origin of the right coronary artery ostium, ischemia and death were attributed to the ostial lesion in one.
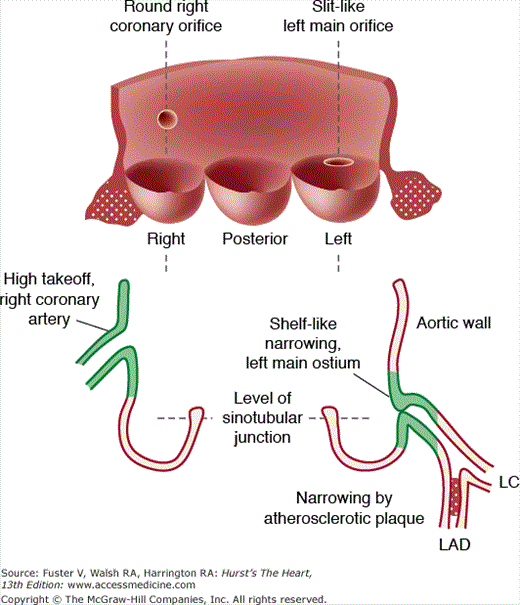
Figure 55–7.
Diagram showing high takeoff position of the right coronary artery and the nonatherosclerotic fibrous ridge occluding the left main coronary ostium. LAD, left anterior descending; LC, left circumflex. Reproduced with permission from Foster et al.11
Nonatherosclerotic causes of coronary ostial narrowing include syphilis,15 Takayasu disease (pulseless disease),16 fibromuscular hyperplasia associated with methysergide therapy,17 aortic valve surgery with or without coronary artery cannulation,18 and ostial valve-like ridges (see Fig. 55–7). A nonatherosclerotic fibrous shelf-like ridge can project from the wall of aorta into the left main ostium.13 It may have been responsible for chronic ischemia and myocardial necrosis. Baroldi15 summarized the other rare diseases that may narrow or occlude the coronary ostia as follows: (1) a nonatheromatous, calcific protrusion from the sinotubular junction into the right or left ostium; (2) saccular aneurysm of the aorta; (3) aortic dissection extending into the coronary ostium—the right ostium being involved more commonly than the left; (4) supravalvular aortic stenosis with severe intimal thickening; (5) obliteration of the ostium as a result of adhesion of the free edge of an aortic cusp to the aortic wall above the coronary ostium; (6) occlusion by embolus (see Coronary Artery Emboli section); and (7) occlusive fibroelastosis.
Anomalous Origin of One or Two Coronary Arteries from the Pulmonary Trunk
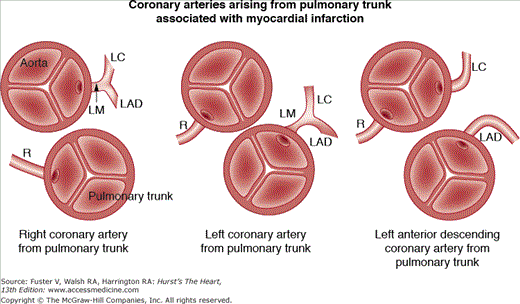
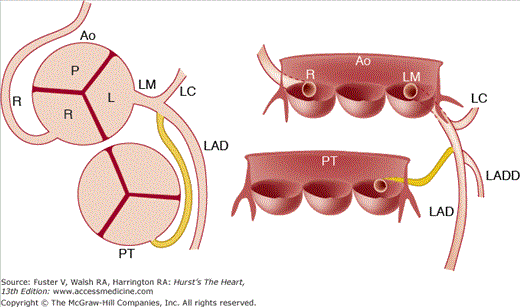
Anomalous origin of a coronary artery from the pulmonary trunk (Figs. 55–9 and 55–10) may be responsible for myocardial ischemia and infarction in infants and children. In >90% of cases,6,7 the left main is the anomalous artery; thus, the anteroseptal and anterolateral left ventricular myocardium may be at jeopardy for injury. Asymptomatic older patients with this coronary anomaly are usually found when they present with an abnormal electrocardiogram (ECG), a systolic murmur, or sudden death.7 The murmur and abnormal ECG are the result of papillary muscle dysfunction and/or anteroseptal myocardial wall damage.
Figure 55–9.
Anomalous origin of one or two major epicardial coronary arteries from the pulmonary trunk. LAD, left anterior descending; LC, left circumflex; LM, left main; R, right cusp. Reproduced with permission from Waller.1
Figure 55–10.
Anomalous origin of the left main (LM) coronary artery from the pulmonary trunk causing acute myocardial infarction in an infant. Of interest, both the anomalous LM and normal right coronary arteries arise in high-takeoff positions from the pulmonary trunk and aorta (Ao), respectively. LAD, left anterior descending; LC, left circumflex; PT, pulmonary trunk; R, right cusp or right coronary artery.
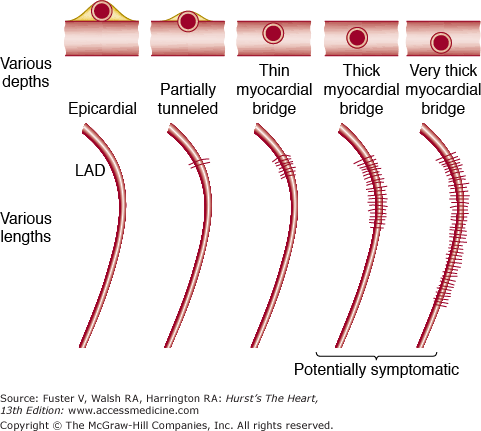
Myocardial Bridges (“Tunneled” Epicardial Coronary Artery)
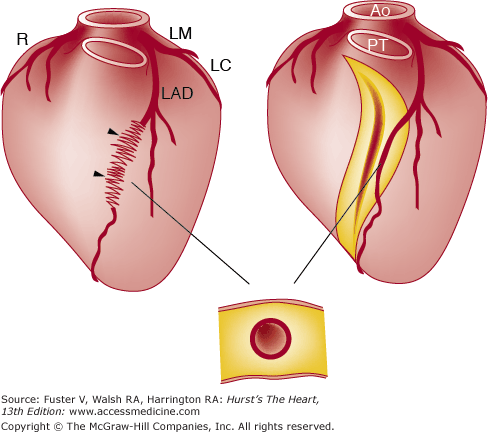
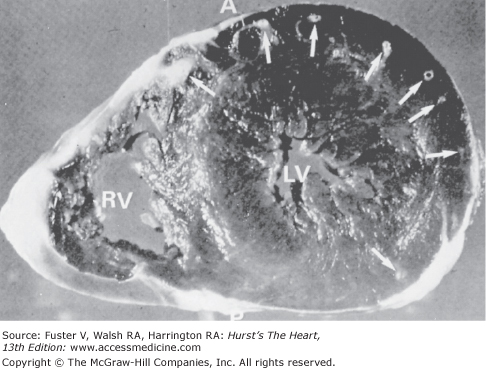
The coronary arteries may dip into the myocardium for varying lengths and then reappear on the heart’s surface (Figs. 55–11, 55–12, 55–13, 55–14, 55–15, 55–16, 55–17, 55–18). The muscle overlying the intramyocardial segment of the epicardial coronary artery is termed a myocardial bridge, and the artery coursing within the myocardium is called a tunneled artery (see Figs. 55–11, 55–12, 55–13).16-25 Tunneled coronary arteries have long been recognized anatomically,16 but suggested associations between myocardial ischemia and myocardial bridges have heightened their clinical relevance.17
Figure 55–11.
Left: Diagram showing tunneled left anterior descending (LAD) coronary artery (arrowheads). Right: Opened left ventricle showing intramyocardial segment. Below: Transverse section of left ventricular wall showing tunneled coronary artery surrounded by myocardium. Ao, aorta; LC, left circumflex; LM, left main; PT, pulmonary trunk. Reproduced with permission from Waller.1
Figure 55–12.
Diagram showing segments of tunneled and nontunneled epicardial coronary artery with changes during ventricular systole and diastole. Ao, aorta; LV, left ventricle; RV, right ventricle. Reproduced with permission from Waller.9
Figure 55–13.
Tunneled epicardial coronary arteries. Two examples of tunneled left anterior descending coronary arteries. Each artery is surrounded by myocardium. Reproduced with permission from Waller.1
Figure 55–14.
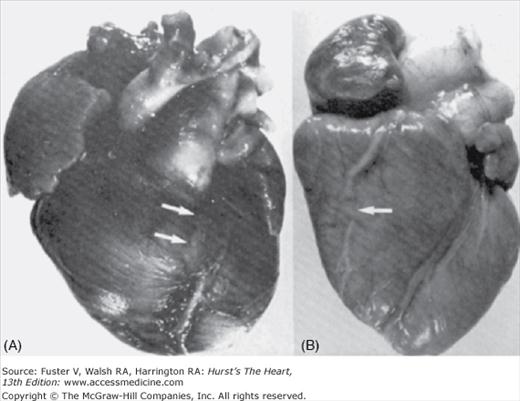
Transverse section of ventricular myocardium showing the “arcade” of tunneled epicardial coronary arteries (arrows). A, anterior; LV, left ventricle; P, posterior; RV, right ventricle. Reproduced with permission from Waller.1
Figure 55–15.
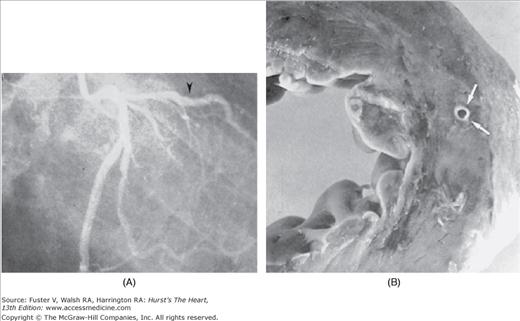
Tunneled epicardial coronary artery. A. Coronary angiogram showing tunneled segment of epicardial coronary artery. B. Corresponding segment of tunneled left circumflex coronary artery (arrow). Reproduced with permission from Waller.1
Figure 55–16.
Tunneled left anterior epicardial coronary arteries from two newborn infants. A. Tunneled left anterior descending. B. Tunneled marginal branch of right coronary artery. Reproduced with permission from Waller.1
Figure 55–17.
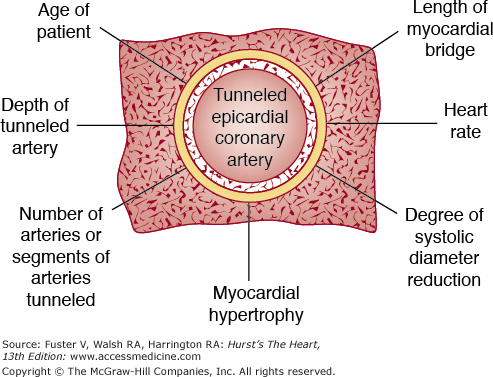
Diagram showing some of the clinical and anatomic factors in a tunneled epicardial coronary artery. Reproduced with permission from Waller.1
Figure 55–18.
Diagram showing morphologic variations in tunneling (length of tunneled segment, depth of tunneled segment). LAD, left anterior descending. Reproduced with permission from Waller.1
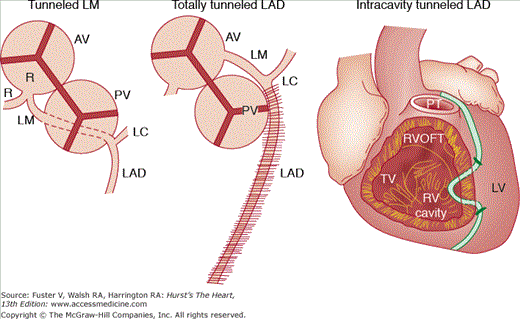
Tunneled coronary arteries are presumed to be congenital in origin. At least three factors are postulated to account for differences between the high frequency of tunneled major coronary arteries observed at necropsy and the lower frequency of tunneled coronary arteries observed angiographically18,24 or associated with symptoms of myocardial ischemia (18%): (1) length of the tunneled coronary segment, (2) degree of systolic compression, and (3) heart rate. Longer tunneled segments of coronary arteries, more severe systolic diameter narrowing of the tunneled segment,17 and tachycardia may contribute to the production of myocardial ischemia with myocardial bridging (see Figs. 55–17 and 55–18). The length of coronary tunneling may not always be an important factor in causing myocardial ischemia, as three cases with left main intramyocardial tunneling of >40 mm have been described without evidence of myocardial ischemia17 (Fig. 55–19).
Figure 55–19.
Diagram showing extremes of tunneled coronary arteries: left main (LM) tunneled through the ventricular septum, total length of the left anterior descending (LAD) located within the myocardium, and tunneled segment of LAD becoming intracavitary. AV, aortic valve; LC, left circumflex; LV, left ventricular; PT, pulmonary trunk; PV, pulmonary valve; R, right cusp or right coronary artery; RV, right ventricle; RVOFT, right ventricular outflow tract; TV, tricuspid valve. Reproduced with permission from Waller.1
Coronary Artery Fistula
A coronary artery fistula is an abnormal communication between an epicardial coronary artery and a cardiac chamber, major vessel (vena cava, subpulmonary veins, pulmonary artery), or other vascular structure (mediastinal vessels, coronary sinus) (Fig. 55–20).6,26-33 This infrequent abnormality can occur at any age and is the most important hemodynamically significant coronary artery anomaly.6,26-33 Many are small and found incidentally during coronary arteriography, whereas others are identified as the cause of a continuous murmur, myocardial ischemia with angina, acute myocardial infarction, sudden death, coronary steal, congestive heart failure, endocarditis, stroke, arrhythmias, coronary aneurysm formation (rupture, emboli), or superior vena cava syndrome.26-30 Of >33,000 patients undergoing coronary arteriography, coronary artery fistula occurred in 0.1%, whether as a result of congenital or acquired causes (Table 55–3). Fistulas from the right coronary artery are more common than from the left coronary artery, and >90% of the fistulas drain into the venous circulation. Most fistulas are single communications, but multiple fistulas have been identified. The natural history of coronary artery fistulas is variable, with long periods of stability in some and sudden onset or gradual progression of symptoms in others. Spontaneous closure is uncommon. Surgical repair of the fistula may be recommended for symptomatic patients and for those asymptomatic patients at risk for future complications (eg, coronary steals, aneurysms, large shunts). Transcatheter embolization of fistulas has been reported. Direct connection between a major epicardial coronary artery and a cardiac chamber or major vessel is the most common hemodynamically significant coronary artery anomaly (see Fig. 55–19). Myocardial ischemia has been documented in some patients with coronary artery fistulas who have no evidence of coronary atherosclerosis.6
Figure 55–20.
Diagram showing coronary artery fistula connecting pulmonary trunk and left anterior descending (LAD) artery. It originally was misdiagnosed as an anomalous coronary artery. Ao, aorta; L, left; LC, left circumflex; LADD, diagonal branch of LAD; LM, left main; PT, pulmonary trunk; R, right.
|
|
Treatment of symptomatic, clinically recognized myocardial bridges has involved calcium channel blockers (control of tachycardia and antispasmodic effects) and surgery. Several cases have now been reported25 in which supra-arterial myotomy (release of myocardial bridge, excision of myocardial bridge) has resulted in relief of symptoms and improvement in previously abnormal nuclear imaging tests. High-frequency intraoperative echocardiography has been used to image the intramyocardial coronary artery before and after surgical release.25
Coronary Aneurysms
Congenital coronary artery aneurysms are found most commonly in the right coronary artery.33 Abnormal flow patterns within the aneurysm may lead to thrombus formation, with subsequent vessel occlusion, distal thromboembolization, and myocardial infarction.34 In general, angina pectoris or acute myocardial infarction that is present in patients who are younger than 20 years of age should prompt suspicion of a congenital coronary artery anomaly or a congenital coronary artery aneurysm.33 Coronary artery aneurysms are found in approximately 1.5% of patients studied at necropsy or by coronary arteriography.35-41
Coronary artery aneurysms, which may be multiple, can be congenital or the result of atherosclerosis, trauma, angioplasty, atherectomy, laser procedures, arteritis (including syphilis), mycotic emboli, mucocutaneous lymph node syndrome (Kawasaki disease), systemic lupus erythematosus, or dissection (spontaneous or secondary) (see Table 55–3). Atherosclerosis-induced aneurysms are thought to result from primary thinning and/or destruction of the media and may represent up to 50% of the causes (Table 55–4). Angioplasty, atherectomy, vasculitis, and arteritis may also damage the arterial wall (media) and lead to coronary aneurysms.37,40,41
| Atherosclerosis (destruction of coronary media) |
| Trauma |
| Angioplasty |
| Atherectomy |
| Laser |
| Arteritis (including syphilis, lupus erythematosus) |
| Mycotic emboli |
| Mucocutaneous lymph node syndrome (Kawasaki disease) |
| Congenital |
| Dissection |
| Neoplasm |
| tissue disorders (Ehlers-Danlos, Marfan) |
Recent reports indicate unusual clinical presentations of large (giant) coronary artery aneurysms: intramural thrombus,42 fistulae,43-49 post coronary stenting,50-54 aneurysm mimicking intracardiac mass or compressing the heart,55-57 and aneurysm producing superior vena cava syndrome.58 An unusual subset of coronary artery aneurysms involving only the left main artery has been reported in adults.59-62 The left main artery has the least involvement of the major coronary arteries with a prevalence of 0.1%. The etiology of this subset includes atherosclerotic and traumatic causes.
Coronary Artery Emboli
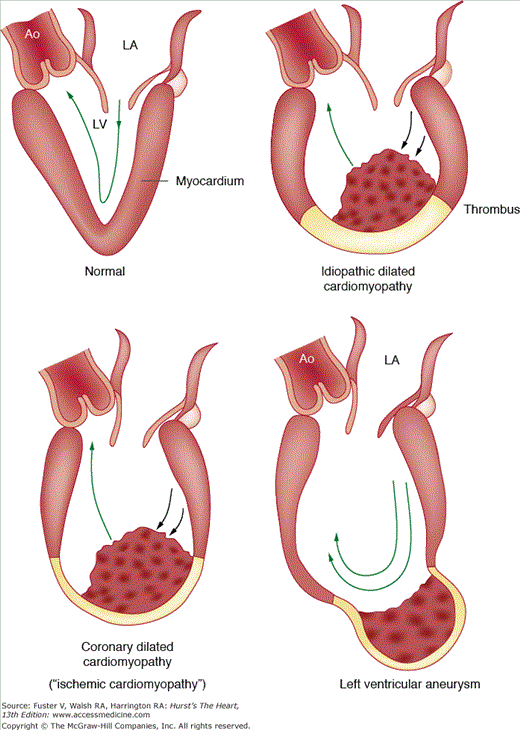
Coronary arterial emboli (Figs. 55–21, 55–22, 55–23, 55–24, 55–25) are clinically suspected in patients who develop severe chest pain with acute myocardial infarction in the presence of a prosthetic left-sided valve, active infective endocarditis, native left-sided valve stenosis, atrial fibrillation, known left ventricular thrombus, left ventricular aneurysm, dilated cardiomyopathy (see Fig. 55–22), known cardiac tumor, or during cardiac catheterization or cardiac surgery.
Figure 55–21.
Coronary artery embolus. Fibrin-platelet thrombus occluding the left anterior descending coronary artery. The source of the embolus was not established, but the patient had recently undergone cardiac surgery. Reproduced with permission from Waller.1
Figure 55–22.
Diagram showing factors associated with emboli from left ventricular (LV) thrombus in three conditions: (1) idiopathic dilated cardiomyopathy (IDC); (2) coronary dilated cardiomyopathy (CDC); and (3) left ventricular aneurysm. Thrombus protruding into the left ventricular (LV) cavity (IDC, CDC) is more likely to embolize than thrombus protected within the sac of an LV aneurysm. Underlying myocardial contraction is more likely to propel thrombus out of the LV outflow tract than paradoxical motion of LV aneurysm. Ao, aorta; LA, left atrium; MV, mitral valve. Reproduced with permission from Cabin HS, Roberts WC. Left ventricular aneurysm, intraaneurysmal thrombus and systemic embolus in coronary heart disease. Chest. 1980;77:586-590.
Figure 55–23.
Coronary artery embolus. A. Postmortem coronary angiogram showing normal epicardial coronary arteries except for sudden cutoff of the distal third of the left anterior coronary artery (arrow). B. Portion of anterior left ventricle and proximal left anterior descending coronary artery showing normal artery. C. Site (arrow) of embolic occlusion of the left anterior descending coronary artery. The remaining distal left anterior descending, right, left circumflex, and left main coronary arteries were normal. Reproduced with permission from Waller.1
Figure 55–24.
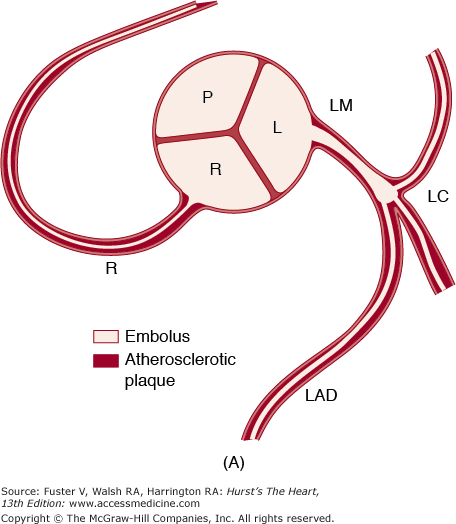
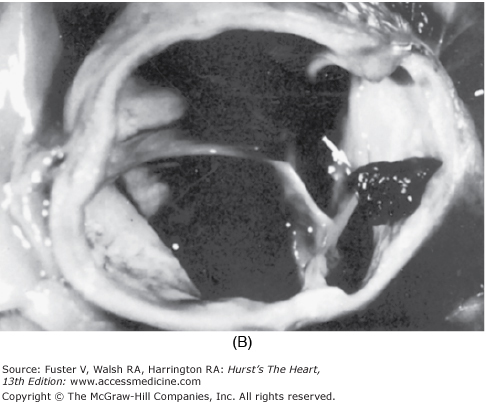
Coronary artery embolism. A. Diagram showing location and extent of occlusion of the left main (LM) coronary artery by an embolus. B. Photograph of aortic root showing embolus protruding from the LM coronary ostium (arrow). L, left; LAD, left anterior descending; LC, left circumflex; P, pulmonary; R, right. Reproduced with permission from Waller et al71
Figure 55–25.
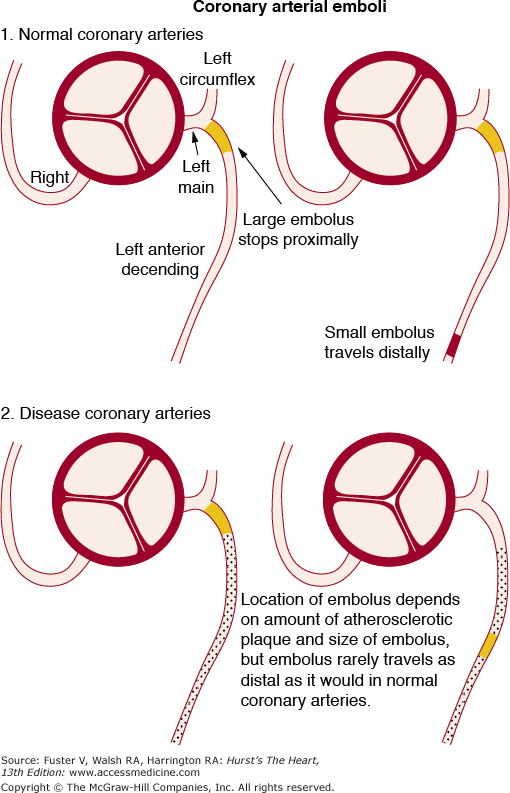
Coronary emboli in normal and diseased coronary arteries. Reproduced with permission from Waller.1
Coronary emboli can be caused by natural, iatrogenic, or “paradoxical” causes (see Figs. 55–21, 55–22, 55–23, 55–24, 55–25). Coronary embolism most often involves the left anterior descending coronary artery. Coronary artery embolism of tumor fragments or thrombus from the surface of tumors is an unusual cause of acute myocardial infarction. Left-sided primary cardiac tumors (eg, myxoma, angiosarcomas, rhabdomyosarcoma, rhabdomyoma, fibrosarcoma, lipoma papillary fibrosarcoma) or metastatic cardiac tumors (eg, primary or metastatic pulmonary tumors, osteogenic sarcoma, renal cell carcinoma) can cause coronary emboli traveling from left atrium or left ventricle to the coronary circulation. Right-sided cardiac primary or metastatic tumors can only produce coronary emboli in the presence of an intracardiac shunt such as a septal defect.
Coronary embolism is suspected as the cause of acute myocardial infarction when, at necropsy, the zone of necrosis is large but discrete (insufficient time to develop effective collaterals). Embolic coronary artery lesions can resolve completely and spontaneously and provide an explanation for angiographically normal coronary arteries several months following an acute myocardial infarction.
The consequences of coronary embolism depend on two major factors (see Fig. 55–25): the size of the embolus and the size of the lumen of the artery in which it becomes impacted. The smaller the embolus, the greater the chance that it will travel distally to a small coronary arterial segment and the less the likelihood of myocardial infarction or fatal arrhythmia.33 An embolus so small that it travels distally and impacts in a single intramural vessel is probably clinically silent and observed only at necropsy.33,34 An embolus to a previously normal coronary artery is likely to migrate distally and result in localized myocardial infarction because of absence of collaterals. An embolus traveling to a previously diseased coronary artery is more likely to impact proximally. Emboli to the left main coronary arteries are rare but usually fatal (see Fig. 55–24).
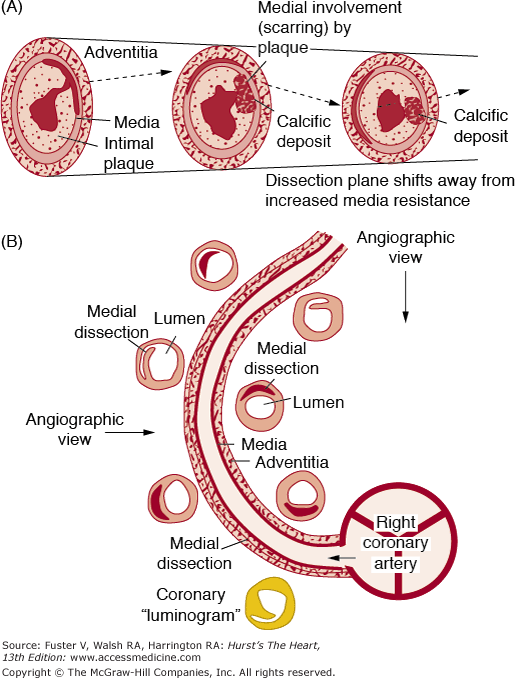
Coronary Artery Dissection
Separation of the media by hemorrhage with or without an associated intimal tear is termed coronary artery dissection. The medial separation forces the intimal-medial layer (wall of true channel) toward the true coronary lumen and produces distal myocardial ischemia/infarction (Figs. 55–26 and 55–27). Coronary artery dissections may be primary63-70 or secondary.71 Secondary coronary artery dissections are more frequent, especially those associated as an extension from aortic root dissection (8%).6 Primary coronary artery dissections may occur spontaneously or as a consequence of coronary angioplasty or angiography, cardiac surgery, or chest trauma (0.3%).71 Most spontaneous coronary artery dissections occur in women who are most commonly postpartum; they may be associated with coronary artery wall eosinophils.72-76 The left anterior descending artery is the one most frequently involved. Systemic hypertension does not appear to provide a significant factor of risk.33 A new cause for spontaneous coronary artery dissection is lysyl oxidase (LOX) deficiency as reported by Sibon et al.77 LOX deficiency has a major role in human arterial wall organization in utero.
Figure 55–26.
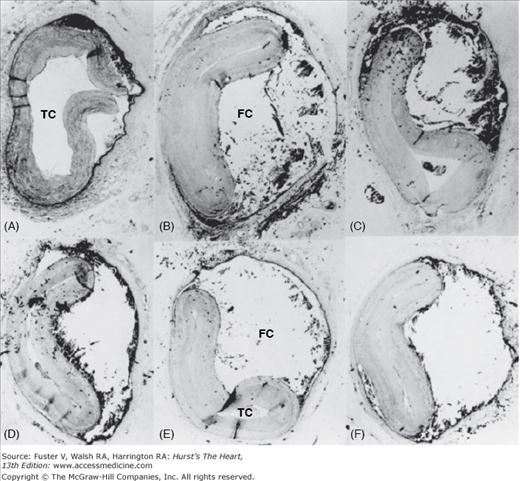
Coronary artery dissection. Serial cross-sections (A-F) showing dissection of the left anterior descending coronary artery. The true channel (TC) is severely compromised by external compression from the false channel (FC) (“dissection channel”). Reproduced with permission from Waller.1
Figure 55–27.
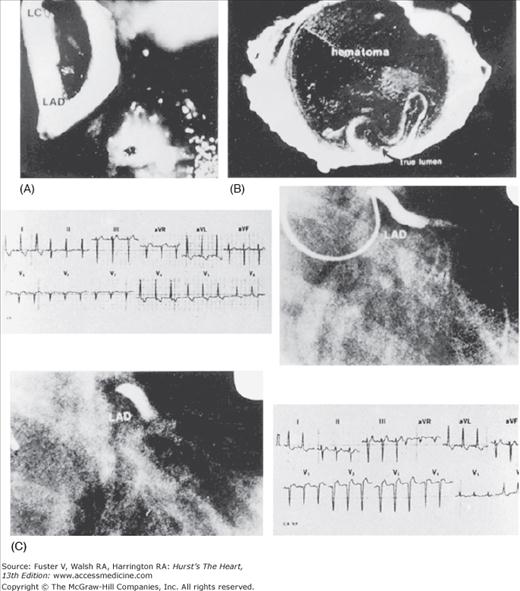
Coronary artery dissection. Occlusion of the left anterior descending (LAD) artery due to dissection. A. The LAD and left circumflex (LC) are seen through the left main artery. B. Cross-section shows that hematoma in false channel severely narrows native (true channel) unobstructed lumen. C. Sequential electrocardiographic and angiographic findings. Reproduced with permission from Isner and Donaldson.72
Spontaneous coronary artery dissection may result in sudden death or acute myocardial infarction and subsequent death. Spontaneous coronary artery dissection that becomes chronic (chronic dissection) may result in congestive heart failure. This circumstance is found primarily in postpartum women.76 In a series reported by DeMaio et al78 and Nishikawa et al,76 75% of cases were diagnosed only at autopsy, and 75% of these patients were women (half of women were postpartum).76 In a few patients with multivessel coronary artery dissection, systemic hypertension was the only association identified. Acute spontaneous coronary artery dissection following fibrinolytic therapy may extend the dissection process or promotion rupture of the false channel because of “engorgement” tearing a thin medial adventitial wall of the false channel. Recognition of the associated causes and conditions of spontaneous coronary artery dissection (including absence of classic coronary atherosclerotic disease risk factors) should alert clinicians to the possibility of coronary dissection producing acute myocardial infarction and use of urgent coronary arteriography instead of automatic infusion of fibrinolytic agents.
Spontaneous dissection of the left main artery represents a unique subgroup of patients.79-81 It is extremely rare and generally fatal.
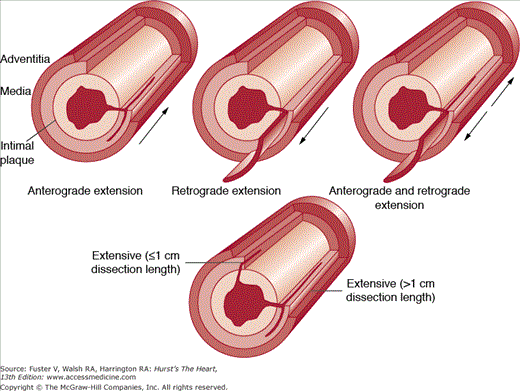
Localized and limited coronary artery dissection (ie, intimal-medial tear) appears necessary for a clinically successful coronary artery balloon angioplasty procedure. Coronary angioplasty dissections viewed in short- or long-axis tomographic images help distinguish dissections that are therapeutic (mechanism) from those that are complications of angioplasty (complications). In the short-axis image, dissection involving >50% of the coronary media circumference has been considered a complication. Similarly, in the long-axis image, dissections (antegrade, retrograde, or both) longer than 1 cm in length have also been defined as a complication of angioplasty (Fig. 55–28). A combination of dissection >50% of short-axis circumference and >1-cm antegrade or retrograde dissection of long-axis length may result in “intussusception” of intimal-medial tissue. Spiral dissections are among the most serious dissection injuries after balloon angioplasty (Fig. 55–29). The spiral dissection as reviewed angiographically appears to alternate from side to side, extending antegrade and retrograde (see Fig. 55–29A), or it has an unaltered dissection course but appears alternating from limited angiographic views (see Fig. 55–29B).
Figure 55–28.
Diagram showing morphologic definition of coronary artery dissections in balloon angioplasty (long-axis plane): localized (mechanism) (1 cm in total dissection length) and extensive (complications) (>1 cm in total length). Reproduced with permission from Waller BF, Orr CM, Pinkerton CA, Van Tassel J, Peters T, Slack JD. Coronary balloon angioplasty dissections: the good, the bad, and the ugly. J Am Coll Cardiol. 1992;20:701-706.
Figure 55–29.
Diagram showing pathologic change accounting for angiographic appearance of coronary artery “spiral” dissection. A. Alteration in course of dissection. B. Angiographic appearance of unaltered course of dissection. Reproduced with permission from Waller BF, Orr CM, Pinkerton CA, Van Tassel J, Peters T, Slack JD. Coronary balloon angioplasty dissections: the good, the bad, and the ugly. J Am Coll Cardiol. 1992;20:701-706.
Coronary Artery Spasm
Coronary artery luminal narrowing produced by spasm has been associated with angina pectoris, acute myocardial infarction, and sudden death.82-100 [see Myocardial Bridges (“Tunneled” Epicardial Coronary Artery)]. Despite the extensive clinical information about coronary artery spasm, relatively few necropsy data are available.82-87 Smooth muscle cells in the coronary artery wall may contract in response to various neurologic and pharmacologic stimuli and temporarily reduce the vessel lumen. Specific pathogenesis of this disorder is unknown.84 Enhanced α-adrenergic tone90 and various vasoactive substances, such as histamine, catecholamines, prostaglandins, and thromboxane,88-90 are presently thought to be relevant factors. Necropsy findings have been reviewed in 13 previously reported cases and in 3 new cases (Figs. 55–30 and 55–31).84-93
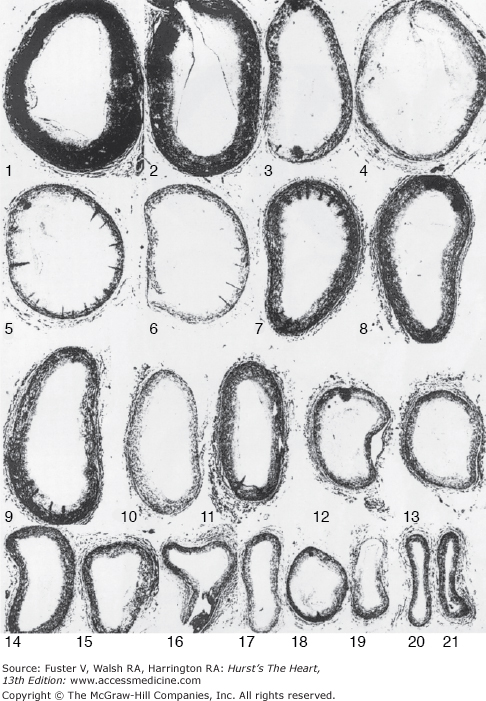
Figure 55–30.
Coronary artery spasm. Composite of coronary artery cross-sections of a patient with coronary spasm during life. Clinical spasm involved segments 3 to 7. Severe atherosclerotic plaque is seen in 8 of the 21 segments. Reproduced with permission from Roberts et al.84
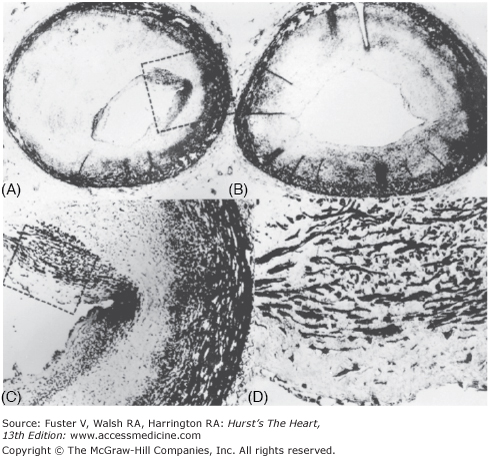
Figure 55–31.
Coronary artery spasm. A and B. Histology sections of the left anterior descending coronary artery at the approximate site of spasm showing severe luminal narrowing. C and D. Higher magnifications of the internal plaque showing the predominance of smooth muscle cells. Reproduced with permission from Roberts et al.84
Most of the 13 previous patients with clinical evidence of spasm had significant fixed coronary luminal narrowing caused by atherosclerotic plaque, although coronary angiograms during life did not recognize these lesions found at necropsy.72,93 In one of the original patients described by Prinzmetal et al,82 both major epicardial coronary arteries were “markedly sclerotic,” and the “posterior coronary artery” was 80% narrowed. Of the subsequent 12 necropsy patients, 10 had at least one major artery severely narrowed by atherosclerotic plaque at necropsy.82-85 The three necropsy patients with clinical spasm72,84 all had severe luminal coronary narrowing by atherosclerotic plaque at least in the artery in which spasm had been demonstrated during life (see Figs. 55–30 and 55–31). In general, histologic sections of the left anterior descending artery at the site of spasm disclosed luminal concentric plaque that had a predominance of smooth muscle cells, suggesting that the lesion may have been responsive to pharmacologic and neurologic stimuli compared with “garden-variety” fibrotic and calcified atherosclerotic plaque (see Fig. 55–31). In a patient with normal angiograms and documented myocardial infarction, “intimal ridges” were observed on postmortem angiography; these were interpreted as evidence of spasm.92
Similar ridges have been noted at necropsy in a patient with coronary artery spasm.93 Histology of the ridges disclosed typical atherosclerotic plaque,101 suggesting that varying degrees of dynamic muscular contraction may be superimposed upon fixed atherosclerotic lesions, presumably related to the amount of smooth muscle present. Coronary artery smooth muscle depletion (“medial attenuation”), which accompanies advanced degrees of luminal narrowing by atherosclerotic plaque, suggests diminished potential for coronary wall spasm.101 It has been recently suggested that medial “contraction” bands may represent a morphologic-histologic marker for arteries that have spasm during life.102
Eccentric atherosclerotic plaques have a segment of disease-free wall with preserved media that presumably has the potential for spasm [see Myocardial Bridges (“Tunneled” Epicardial Coronary Artery)].103 In patients with clinical coronary spasm, unstable and stable angina pectoris, and episodes of silent myocardial ischemia, where 448 segments were narrowed by >75% in cross-sectional area by plaques, 15% of these segments had a variable arc of disease-free wall with normal media. Other studies have found a similar percentage (15%-20%) of normal coronary wall in 70% of cases studied.104,105 This disease-free coronary segment represents a site of “vasospastic potential” and could convert a hemodynamically insignificant lesion of <50% cross-sectional area into a hemodynamically significant one of >75% narrowing.