Introduction
There are three primary causes of aortic stenosis (AS): congenitally bicuspid valves with superimposed calcification, rheumatic valve disease, and calcific disease of a trileaflet valve. Calcific AS is the most frequent expression of valvular heart disease in the Western world, is the leading indication for valve replacement, and its prevalence increases with increasing age. Community data document the prevalence of moderate or severe AS at 0.6%, 1.4% and up to 4.6% in patients aged 55–64, 65–74, and ≥75 years, respectively.1 Surgical aortic valve replacement (AVR) is the preferred treatment strategy for symptomatic patients, but has limitations in the presence of co-morbidities, including advanced age, which increase the operative risk.
Pathophysiology and natural history
The early valve lesions of AS show features similar to atherosclerosis with lipid accumulation and accompanying inflammatory cells and calcification. Myofibroblasts play a central role in the process, and it is believed these cells differentiate into an osteoblast-like cell phenotype which in turn promotes formation of the calcified nodules and bone.2 The calcification process occurs over decades and causes reduced leaflet mobility, which leads to increased transvalvular pressure gradients, left ventricular outflow obstruction, and systolic pressure overload. The left ventricular wall thickens to maintain chamber size, wall stress and ejection fraction,3 which is both beneficial and detrimental. Hypertrophied myocardium has less coronary flow per gram and less vasodilator reserve which makes it vulnerable to ischemia, even in the absence of epicardial coronary disease.4–6 Ischemic stress can be greater in the hypertrophied heart, which must be considered during transcath-eter aortic valve replacement (TAVR) where transient left ventricular outflow obstruction and rapid pacing are pro-cedurally required.
The natural history of adult AS consists of a prolonged latent period during which morbidity and mortality are very low. As the calcification process continues, the transvalvular gradient increases and eventually the cardinal symptoms of AS are experienced: angina, syncope and heart failure. The outlook changes dramatically once symptoms are present, and average survival is 2–3 years.7–9 Progression from mild AS to severe AS is variable, but once moderate stenosis is present the average rate of progression is a decrease in aortic valve area (AVA) of 0.1cm2/ year.10 Once symptoms are present the risk of sudden death is high and AVR must be considered.11
Aortic valve replacement indications and risk
Surgical AVR may offer survival and symptom benefit, and can be performed at low risk with excellent and durable results in most patient groups. The current American College of Cardiology (ACC)/American Heart Association (AHA) valvular heart disease guideline recommendations for AVR are provided in Table 56.1 and, simply stated, give a Class I recommendation to surgical aortic valve replacement for patients with severe AS in combination with symptoms, left ventricular ejection fraction (LVEF) <50%, or concomitant coronary bypass, aortic or heart valve surgery. In the developing arena of transcatheter aortic valve replacement (TAVR), such guidelines do not exist, and the understanding of surgical risk is fundamental to the process of patient selection.
Table 56.1 ACC/AHA recommended indications for AVR in patients with AS37
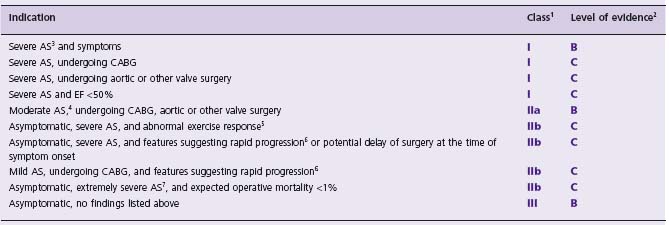
ACC, American College of Cardiology; AHA, American Heart Association; AVR, aortic valve replacement; AS, aortic stenosis; CABG, coronary artery bypass grafting; EF, ejection fraction; AVA, aortic valve area.
1 Definitions for recommendation:
Class I: Conditions for which there is evidence and/or general agreement that the procedure or treatment is beneficial, useful, and effective.
Class II: Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment: IIA Weight of evidence/opinion is in favor of usefulness/efficacy; IIB Usefulness/efficacy is less well established by evidence/opinion.
Class III: Conditions for which there is evidence and/or general agreement that the procedure/treatment is not useful/effective and in some cases may be harmful.
2 Definitions for level of evidence:
A: Data derived from multiple randomized clinical trials.
B: Data derived from a single randomized trial or non- randomized studies.
C: Only consensus opinion of experts, case studies or standard of care.
3 Severe AS: AVA <1 cm2, AVA index <0.6 cm2/m2, mean gradient >40 mmHg, and/or jet velocity >4 m/s.37
4 Moderate AS: AVA 1.5–1 cm2, mean gradient 25–40 mmHg, and/or jet velocity 3–4 m/s.37
5 Development of symptoms or asymptomatic hypotension.
6 Age, calcification, and CAD.
7 AVA < 0.6 cm2, mean gradient > 60 mmHg, and jet velocity >5 m/s.
Among those centers reporting within the Society of Thoracic Surgeons (STS) database (1998–2005) the average perioperative mortality was 3–4% for isolated AVR, and 5–7% for AVR plus coronary artery bypass grafts (CABG).12 In patients ≥70 years of age, other large series report a 5–15% operative mortality for isolated AVR, and rates are higher in the presence of co-morbidities or the need for additional cardiac procedures.13–20
Beyond mortality, morbidity is a significant risk with surgical AVR. For very elderly patients, postoperative stay is more than tw weeks, most are discharged to nursing care or rehabilitation facilities, and rehospitalization within one month is required for >20%.21,22 Nevertheless, some series show excellent outcomes.23
The increased risk of morbidity and mortality may lead patients, or their physicians, to hesitate when considering surgical options. Iung et al24 performed a cross-sectional survey of patients with valvular heart disease presenting to 92 European hospitals or clinics over a three-month period in 2001, and found that 9.8% of those with aortic stenosis who had guideline indications for surgery were not offered intervention. Subsequently they looked at elderly patients with AS (age >75 yrs), and found that 33% of those with indications for surgery were not offered intervention.25 Others found that 40–60% of elderly patients with severe symptomatic AS were not offered intervention.26,27 Patient refusal of surgery has been a consistent reason for conservative management despite the one-, five-and 10-year survivals of 60%, 32%, and 18% respectively of medical therapy.28
The growing series of successful TAVR procedures have been performed in an elderly cohort of “high-risk” or non-surgical candidates. This classification has been based on the clinical opinion of senior surgeons in high-volume centers and the use of objective risk calculators as defined by the European System for Cardiac Operative Risk Evaluation (EuroSCORE)29,30 and/or the Society of Thoracic Surgeons (STS) database.31 Both EuroSCORE and STS provide an estimate of mortality at 30 days or hospital discharge for patients undergoing CABG and/or valve surgery.
The EuroSCORE model considers 17 clinical risk factors, and was developed in a 13 302 patient cohort with validation in a 1479 patient cohort. It was subsequently tested in a North American cohort of 401 684 patients where the predicted mortality was almost identical to the observed mortality (predicted 3.994%, observed 3.992%). The STS model was developed from the STS National Adult Cardiac Surgery Database which collects information on cardiac surgery morbidity and mortality from 497 participating member sites throughout the United States.31 The 11 STS models predict both 30-day mortality and major morbidity, are updated and reported annually, and allow comparisons between the observed and predicted outcomes in an effort to improve quality of care. An alternative risk model for patients undergoing valve surgery has been published by Ambler et al.32 It was developed and validated using the database of the Society of Cardiothoracic Surgeons of Great Britain and Ireland, but remains less well utilized. All groups have facilitated risk calculations by providing on-line calculators: 66.89.112.110/STSWebRiskCalc/, euros-core.org/calc.xhtml and www.ucl.ac.uk/stats/research/riskmodel/.
An objective assessment of individual risk has been commonly used to define TAVR eligibility.33 Published case series have seen average logistic EuroSCOREs >20.34,35 Furthermore, the inclusion criteria for upcoming randomized trials will likely have an objective risk measurement, with possible thresholds being in the range of logistic EuroS-CORE >20 or STS score >10.
Percutaneous balloon aortic valvuloplasty (PBAV) was first performed by Cribier in 1985, with the first three patient series published in 1986.36 Subsequently there have been many reports of the effectiveness and durability of PBAV, and in reviewing the literature for this chapter we collected 50 publications documenting results from over 2400 patients. Most procedures were performed in elderly patients with calcific AS who were considered either non-operative candidates or of high operative risk. All reports are case series (Level C1), and PBAV has never been subjected to a large-scale randomized trial. The discussion below is not a formal systematic review, but is based on a comprehensive and representative sample of English-language publications. We will not discuss the evidence for PBAV in young patients with congenital AS where it remains a Class I therapeutic option.37
The 27-center Mansfield Investigational Device Exemption Protocol employed standardized protocols with definite inclusion and exclusion criteria to study aortic valvuloplasty.38 Patient recruitment occurred from December 1986 through November 1987. The data collected from 492 patients undergoing PBAV were pooled to create the Mansfield Scientific Balloon Aortic Valvuloplasty Registry.38–45 Subsequently, the NHLBI Balloon Valvuloplasty Registry was organized, and enrolled 674 patients from 24 centers between 1987 and 1989, generating reports on early and late procedure results.46,47 The Cribier group in France48–52 and the Duke University Medical Center group53–55 have also contributed significant numbers of patients to this literature.
To support PBAV as a therapeutic option, we must consider the ability of the procedure to do what it claims: reduce the transaortic pressure gradient. Table 56.2 outlines the changes in AVA and mean transaortic gradient (MG) reported in a variety of series. Most reports show the AVA increases to between 0.8 and 1 cm2, and the largest series found AVA increased <0.4cm2 in 77% of patients.47 However, in 13% of this cohort there was either no change or <0.1cm2 improvement in AVA. Similarly, 13% of the Mansfield group had an unsuccessful procedure (procedural/in-hospital death, AVR within seven days, or <40% change in AVA).42 It is clear that PBAV is successful in reducing the transaortic gradient in most, but not all patients with critical AS secondary to calcific disease.
Table 56.2 Summary of hemodynamic effectiveness and procedural complications of percutaneous aortic balloon valvuloplasty
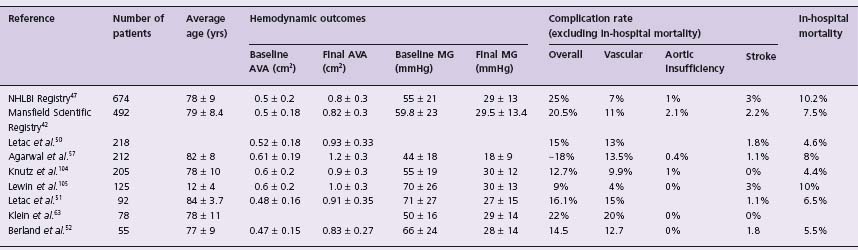
AVA, aortic valve area; MG, mean transaortic gradient.
Another measure of procedural success is symptom status, and all series report significant initial improvement. We present a representative sample of the available data in Table 56.3. The NHLBI registry found symptom improvement in 75% of survivors at 30-days, and 60–85% of survivors report symptom improvement at follow-up out to 24 months. Davidson et al [56] report LVEF was the only independent predictor of overall symptom improvement at 3 months, and found less than 50% of those with an ejection fraction (EF) < 45% had symptom improvement. Taken in aggregate, the data suggests the majority of survivors have some initial symptom improvement.
Table 56.3 Summary of studies reporting symptom response to percutaneous aortic balloon valvuloplasty, sorted by duration of follow-up
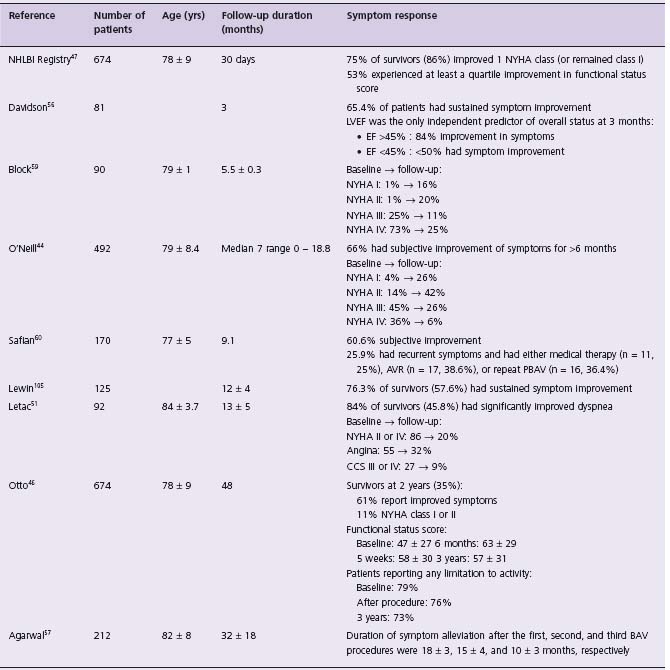
NYHA, New York Heart Association; AVR, aortic valve replacement; LVEF, left ventricular ejection fraction; LV, left ventricle; PBAV, percutaneous balloon aortic valvuloplasty; PG, peak gradient.
However, the result is not durable, and most patients will see symptom recurrence with time. Agarwal et al57 report that the mean duration of symptom relief after PBAV was 18 ± 3 months. Upon repeat investigation, most patients with recurrent symptoms are found to have restenosis (defined as ≥ 50% loss in the AVA gained by PBAV), and this is assumed to be the cause of recurrent symptoms. The rate of restenosis has been reported to vary between 25% and 47% at six months58–60 and up to 80% at 15 months.61 Postprocedure AVA can be influenced by a variety of procedural modifications42,47 and although initially thought to alter prognosis,57–60 these techniques have not been found to change rates of restenosis or survival in larger series.39
Reported periprocedural complication rates range from 9% to 25% (see Table 57.2). The nature of periprocedural complications as reported in the NHLBI Registry can be found in Table 57.4. Procedural morbidity and in-hospital mortality decreases with increased procedural experience and equipment refinements.50,62 Interestingly, the two most recent PBAV series report periprocedural complication rates of 22% and 18%.57,63 Although Klein et al63 do report a trend toward a decrease in procedural complications over time, these complication rates are not significantly less than that reported in the Mansfield Registry. Periprocedural stroke and aortic insufficiency are uncommon complications (see Table 56.2).
The final analysis of PBAV must consider its effectiveness in altering the natural history of the disease. Mortality rates address this question directly, and are presented in Table 56.5. One-and two-year survival ranged from 75% to 38% (average 65%) and 28% to 60% (average 35%), respectively. These rates are not significantly different from the one-and two-year survival of elderly patients with untreated AS (60% and 40%, respectively)9,28 and suggest PBAV alone does not alter the natural history of AS for most patients.
Table 56.5 Summary of studies reporting long-term outcome of percutaneous aortic balloon valvuloplasty sorted by duration of follow-up
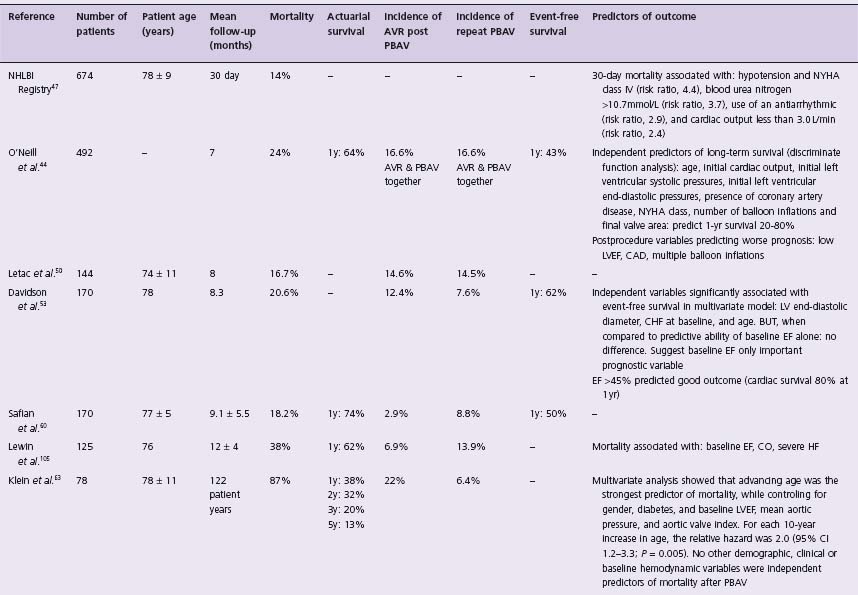
Many authors employed multivariate techniques to identify clinical, historical and/or hemodynamic factors that might be significantly associated with PBAV outcome. These results are presented in Table 56.5 and consistently show older age, low EF, and worse baseline NYHA class are all associated with a poor prognosis. In the largest series, Otto et al defined a “lower risk” subgroup (normal LVEF and mild functional limitation) who had a three-year survival of 36% as opposed to 17% in the rest of the group.46
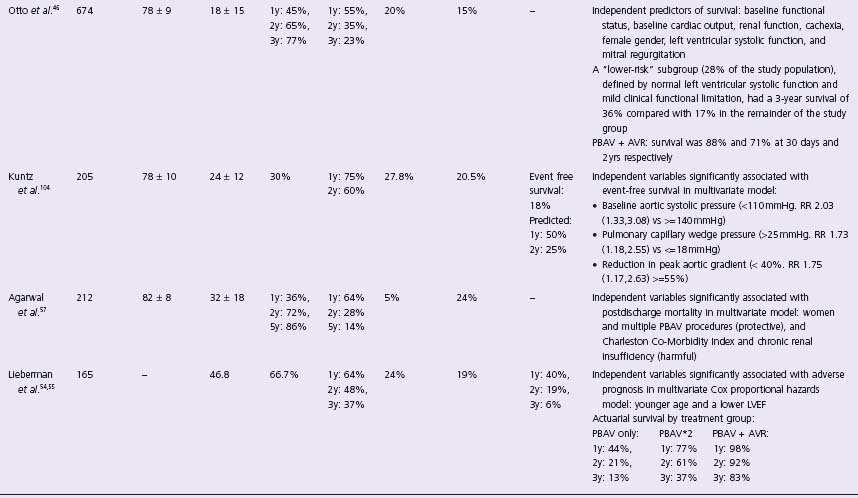
AVR, aortic valve replacement; PBAV, percutaneous balloon aortic valvuloplasty; NR, not reported; m, month; y, year; LV, left ventricle; EF, ejection fraction; NYHA, New York Heart Association; CO, cardiac output; CHF, congestive heart failure.
Given the early benefit but limited durability of PBAV, some investigators proposed that patients with restenosis be considered for repeat valvuloplasty. Table 56.5 provides details on the incidence of such treatment (roughly 5–20%), but the outcomes were most carefully considered in the three separate series.40,49,57 Each series has shown a second procedure is efficacious in reducing AVA and symptoms, but none was able to find factors that would point to an increased durability of a second procedure. Although patients who underwent repeat procedures had higher three-year survival rates than those with a single procedure,54,57 there was a decrease in the duration of symptom relief with each subsequent dilation.57 Several reports have highlighted the success of PBAV followed by AVR where two-year survival can be as high as 92%.54 This is an attractive strategy but has limited applicability as it is unclear how delaying definitive treatment would improve operative risk for the majority of patients. In fact, the AHA/ACC guidelines give a Class III recommendation to the concept of PBAV as an alternative to AVR.37
Despite the problems with PBAV, the current AHA/ ACC guidelines recognize the possibility that it may have a limited therapeutic role, and the following situations are given Class IIB recommendations.
1 Aortic balloon valvotomy might be reasonable as a bridge to surgery in hemodynamically unstable adult patients with AS who are at high risk for AVR.
2 Aortic balloon valvotomy might be reasonable for palliation in adult patients with AS in whom AVR cannot be performed because of serious co-morbid conditions.37 Additionally, several groups have reported that PBAV can be used to successfully treat patients with cardiogenic shock secondary to severe AS.64,65 Similarly, PBAV can be used to reduce the risk of non-cardiac surgery when a stepwise approach using AVR is not possible.66,67
In an attempt to address the significant problem of limited durability with PBAV, newer studies have focused on the mechanisms of restenosis. PBAV improves leaflet mobility by fracturing calcified nodules and creating cleavage planes within collagenous stroma.68,69 Feldman et al70 suggest that the process of restenosis following PBAV is histologically distinct from that of calcified valves not having undergone PBAV. They found granulation tissue, fibrosis, and ossification present within the leaflets of valves treated with PBAV, suggesting an active process of osteoblast-mediated calcification. This lead Pedersen et al to perform a 20-patient pilot study of external beam radiation applied 3–5 days following PBAV in elderly patients with calcific AS (the RADAR pilot trial).71 They found one-year restenosis rates of 21–30% in a dose-responsive manner. This is substantially less than the 15-month 80% restenosis rates found by Letac et al61 and offers an interesting option for the future of PBAV techniques.
In summary, PBAV is successful in reducing left ventricular outflow obstruction in most patients with severe calcific AS. However, the results are not durable, and the long-term survival resembles that of the natural history of critical AS. We conclude that PBAV currently has a limited role in palliation of some patients with critical AS, and surgical AVR remains the treatment of choice. However, new therapies targeting post-PBAV restenosis offer promise and may lead to expanded indications in the elderly population. Furthermore, the future role of PBAV may be influenced by the development of TAVR as discussed below.
Andersen et al pioneered the development of a balloon-expandable aortic valve, reporting experience with a porcine model in 1992.72 Subsequently, others demonstrated the feasibility of percutaneous prosthetic valve delivery.73–77 In 2000, Bonhoeffer et al described the first-in-man experience of a successfully implanted catheter-based stent valve in a pulmonary conduit.78 In 2002, Cribier et al demonstrated the feasibility of aortic valve implantation in man using an antegrade approach via the femoral vein and transseptal puncture,79 following which we described the development of a retrograde approach via the femoral artery.80 Subsequently, the experience and technology for transcatheter aortic valve replacement (TAVR) have evolved rapidly, and will be discussed below. Like the PBAV literature, the evidence for these procedures is limited to case series (Level C1), but randomized trials are under way.
Balloon-expandable aortic valve
The currently available SAPIEN ™ valve (Edwards Life-sciences LLC, Irvine, CA) consists of three bovine pericar-dial leaflets hand-sewn to a stainless steel, tubular, slotted, balloon expandable stent (Fig. 56.1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


