BOX 73.1Risk evaluation of the patient with possible non-ST segment elevation ACS
High-risk features that support early invasive strategy
- Elevated troponin or CKMB
- ECG ST segment depression or transient ST segment elevation
- Associated heart failure or hypotension
- Life-threatening ventricular arrhythmias
- Refractory myocardial ischemia despite initial medical treatment
Other features that indicate an increased risk for adverse outcomes
- Older age
- Diabetes
- Impaired renal function
- Impaired left ventricular function
- Recent ACS
- Known extensive coronary artery disease
Our patient has no high-risk features that would by themselves indicate high risk. Yet age and the history of diabetes give her a score of 3 according to the scoring system in Figure 73.1. She received dual antiplatelet therapy with aspirin and clopidogrel. An anticoagulant (a heparin, fondaparinux or bivalirudin) was not given.
Case history continued
The blood sample taken at six hours after the patient arrived in the emergency department shows the troponin I has increased to 0.15 ng/L (99 percentile reference level 0.06 ng/L). Would this result change your management?
Comment
The presence of an elevated troponin I level in a patient with symptoms and circumstances compatible with an ACS is objective evidence supportive of the diagnosis of ACS, and indicates:
- a higher risk for recurrent ACS
- benefit from an early invasive strategy of coronary angiography and revascularization when appropriate ^ benefit from immediate use of antiplatelet and anti-thrombotic treatment.
Outcomes related to elevated troponin
The relationship between elevated biomarkers of myocardial necrosis and outcomes has been recognized for many years. The patient with a myocardial infarction and increased biomarkers (transaminases, lactate dehydrogenase (LDH) or creatine kinase (CK)) has substantially increased mortality when compared to the patient presenting with rest pain and no biochemical evidence of infarction, i.e. unstable angina. With the development of troponin as a highly sensitive and specific marker of myocardial injury, approximately 30% of patients with a previously designated diagnosis of unstable angina and normal CK and CKMB have increased troponin. Whereas early mortality relates to both the maximal CKMB and troponin levels, the risk of early myocardial (re-) infarction is more related to low levels of troponin.3 Small increases in troponin I or T in a patient with a clinical history compatible with an acute coronary syndrome indicate increased risk of recurrent ACS and the need for more aggressive treatment. It should be recognized that many other clinical situations such as pulmonary embolism, septic shock, acute heart failure and renal failure are associated with minor degrees of myocardial injury indicated by elevation of troponin, that are not due to thrombotic coronary occlusion. The troponin elevation should be evaluated in the clinical context.
Early invasive strategy or conservative management?
The strategy of early cardiac catheterization and revascularization (when appropriate) reduces early recurrent acute coronary syndromes and long-term mortality when compared to a conservative strategy of cardiac catheterization only if the patient has recurrent ischemia or high-risk non-invasive testing.
Four meta-analyses4–7 have showed that an early (during the index hospitalization) invasive strategy is superior to a selectively invasive approach. A meta-analysis of the seven most recent trials, included 9212 patients and showed that death or myocardial infarction was reduced by 18% (odds ratio (OR) 0.82, 95% confidence interval (CI) 0.72–0.93) over a follow-up period of 17 months (range 6–24 months) with routine early coronary angiography and revascularization (when appropriate), compared to a selective approach5 (Fig. 73.2). An analysis showed improved outcomes, including reduced mortality, were only achieved when coronary stenting and an aggressive antiplatelet strategy were employed.6 The early benefit from an invasive strategy is mainly from a reduction of myocardial infarction. However, long-term mortality was reduced in the FRISC 28 and the RITA 39,10 trials with a 26–30% reduction in 1–5-year mortality compared to patients managed conservatively. High-risk patients with elevated troponin or greater TIMI risk scores benefit the most from the early invasive strategy.11–13
Figure 73.2 Meta-analysis of clinical trials comparing an early invasive with a conservative strategy in the management of patients with NSTE ACS.61
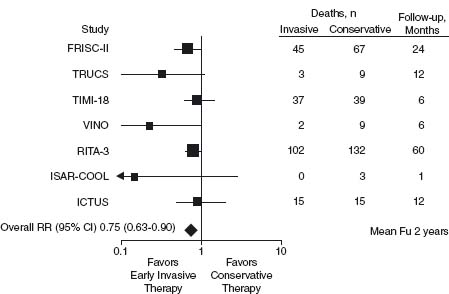
Early revascularization within 48 hours in high-risk patients with non-ST segment elevation (NSTE) ACS is supported by data from the CRUSADE registry14 and the TACTICS/TIMI 18 study.11 Furthermore, the ISAR COOL trial15 indicated that there was no benefit from a 72-hour period of medical stabilization with an aggressive protocol of heparin, acetylsalicylic acid (ASA), clopidogrel and glycoprotein (GP) IIb/IIIa inhibitors compared to immediate cardiac catheterization within six hours. Ischemic events occurred more frequently prior to percutaneous coronary intervention (PCI) in the medical stabilization group despite the vigorous treatment. Hence the current evidence supports early angiography within the first 48 hours in the majority of patients identified as being at high risk. For patients with frequent episodes of ischemia, heart failure or hypotension, even more urgent coronary angiography allows immediate revascularization in very high-risk individuals.
The 2007 ACC/AHA guidelines16 for the management of NSTE ACS have recommended that an initially conservative management strategy can be considered for initially stabilized patients even with high-risk features (Class IIb, Level C). This recommendation is based on the results of the ICTUS trial.17 Patients included had rest pain, elevated troponin and either ECG abnormalities or a known coronary artery disease. The study compared an early invasive management strategy, with coronary angiography and revascularization where feasible within 24–48 hours, with a selectively invasive approach that only progressed to an invasive management with recurrent ischemia or a high-risk stress ECG. In the ICTUS study myocardial infarction was defined as any increase in CKMB beyond the normal range, with no higher threshold for patients undergoing PCI or bypass surgery. During the index hospitalization 98% of the early invasive group and 53% of the selectively invasive group underwent coronary angiography. One year after the index ACS event, 67% of the selectively invasive group had undergone coronary angiography. At one year after the ACS event, there was no difference in the primary outcome of death/recurrent myocardial infarction or unstable angina. The ICTUS patients had a low incidence of cardiovascular events (one-year mortality 2.5%). Almost two thirds of the conservative group underwent coronary angiography and revascularization by the end of the first year. Thus the management strategies mainly differed by the timing of the invasive strategy and the study compared an early invasive with a delayed invasive strategy. It is unlikely therefore that this study alone will change practice.
Antithrombotic and antiplatelet therapy
The vast majority of acute coronary syndromes result from arterial thrombosis occurring with either atherosclerotic plaque erosion that denudes endothelial cells or plaque rupture exposing thrombogenic collagen and tissue factor to the blood. Propagating thrombus may occlude the vessel. However, the size of the thrombus and the activity of intrinsic fibrinolysis may determine whether the vessel remains occluded or is spontaneously recanalized. A high proportion of patients with NSTE ACS have patent culprit coronary arteries when investigated within the first 24 hours. This is in contrast to ST segment elevation ACS (STE ACS) where 80% of patients have an occluded infarctrelated artery. In the patient with NSTE ACS the rationale of therapy is to prevent recurrent ACS due to reocclusion of both the culprit artery and other sites of plaque rupture or vulnerability, by using medications (antiplatelet and antithrombotic agents) and revascularization when feasible and indicated.
Medications to reduce thrombosis are the mainstay of early therapy in patients with acute coronary syndromes. Drugs reducing platelet activation and aggregation such as ASA, clopidogrel and GP IIb/IIIa inhibitors, and agents interfering with blood coagulation such as heparins, fondaparinux and bivalirudin have been shown to reduce recurrent ischemic events when administered in the first few hours after the onset of symptoms. The clinical trials for many agents were performed in an era when cardiac catheterization was performed less frequently and usually much later than has become current practice. Consequently, application of the results to current practice requires interpretation and extrapolation often beyond information provided by the individual trials.
Antiplatelet therapy
ASA was studied in patients with unstable angina and shown to reduce recurrent events both early and over many months. The benefit was observed in patients receiving no antithrombotic treatment and before the days of frequent revascularization. In patients with NSTE ACS the CURE trial18 demonstrated that the addition of clopidogrel 75 mg daily to ASA reduced the primary endpoint (cardiovascular mortality, myocardial infarction or stroke) by a relative 20% (2% absolute risk reduction) over the nine-month period of treatment. Within hours of administration of the 300 mg loading dose, there was a significant reduction of the combined endpoint of cardiovascular death, myocardial infarction (MI) and recurrent severe ischemia. The majority of patients included in the CURE trial had either ECG abnormalities or positive biochemical markers (usually CKMB). However, for the first 3000 patients enrolled, age greater than 60 and a history of coronary artery disease were entry criteria. Across a wide risk spectrum, there was a constant relative risk reduction with clopidogrel. The benefits were achieved with a 1% absolute increase in major bleeding, mainly determined by a 50 g/L fall in hemoglobin or need for transfusion, yet with no increase in life- threatening bleeding. Approximately one-third of patients underwent revascularization after randomization. Patients undergoing both percutaneous coronary intervention19 and coronary artery bypass surgery20 had benefit from pretreatment with clopidogrel before revascularization. However, for the few patients undergoing coronary bypass surgery within five days of receiving clopidogrel, major bleeding was increased 50%.18
The intravenously administered GP IIb/IIIa inhibitors abciximab, tirofiban and eptifibatide were studied in patients with NSTE ACS before clopidogrel was used in the early management of these patients. A meta- analysis of clinical trials that included over 31 000 patients suggested a modest benefit with one fewer death or MI at 30 days per 100 patients treated.21 The greatest benefit was observed in higher risk patients, such as those with troponin elevation,22,23 and diabetes mellitus.24
Currently it is unclear whether the addition of a GP IIb/IIIa inhibitor to dual antiplatelet therapy with ASA and clopidogrel improves outcomes. The ISAR React 2 study evaluated the use of abciximab at the time of PCI in patients with recent NSTE ACS pretreated with clopidogrel. Those receiving both clopidogrel and abciximab and with elevated troponin had a 25% reduction of the combined end-point of death/non-fatal myocardial infarction or urgent target vessel revascularization.
Antithrombotic treatment
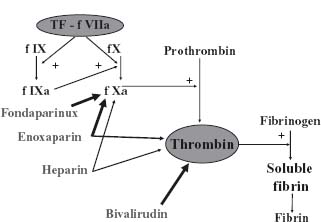
Activation of the coagulation pathway by agents such as tissue factor initiates a positive feedback system activating platelets and resulting in rapid growth and propagation of the thrombus. Control of the coagulation pathway is important to prevent recurrent ischemic events in patients with acute coronary syndromes. Available agents and their interaction with the coagulation pathway are shown in Figure 73.3.
Figure 73.3 The sites of action of the antithrombin agents currently used in the management of patients with NSTE ACS.
Unfractionated heparin (UFH)
An analysis of six clinical trials in patients with NSTE ACS that compared UFH with placebo showed a 33% reduction of death and MI over the short term (OR 0.66, 95% CI 0.44–0.99, P= 0.045).25 When UFH is combined with ASA and compared to ASA alone, there is a trend to a benefit with the combined therapy.26 Studies that compare either UFH or low molecular weight heparin combined with ASA with ASA alone show a 39% reduction of death and MI.
Low molecular weight heparin (LMWH)
When compared with placebo in patients with NSTE ACS, there is benefit from LMWH (death/MI OR 0.34, 95% CI 0.20–0.58, P < 0.0001).27 However in this meta-analysis LMWH was not superior to UFH (OR 0.88, 95% CI 0.69–1.12, P= 0.34). However, individual LMWH preparations may be superior to UFH. When enoxaparin is compared to UFH a meta-analysis showed no difference in mortality outcomes at 30 days (OR 1.00, 95% CI 0.85–1.17). Yet death/MI was reduced 9% by enoxaparin (OR 0.91, 95% CI 0.83–0.99).28 In this analysis there was no increase in bleeding as defined by major bleeding or transfusion needs when enoxaparin was used. A more contemporary use of enoxaparin in the SYNERGY trial29 included 9978 high-risk patients with NSTE ACS destined to undergo very early coronary angiography and revascularization. Patients were randomized to receive either enoxaparin or UFH. Coronary angiogra-phy was performed an average 22 hours after randomization. In the context of aggressive, very early invasive management, enoxaparin was not shown to be superior to UFH. There was a trend to increased major bleeding in patients receiving enoxaparin (OR 1.17, 95% CI 0.99–1.39). As a result of the SYNERGY trial, UFH is preferred over enoxaparin if the patient is likely to go to the catheterization laboratory within the first 24 hours after presentation.
Fondaparinux
The synthetic anti-factor Xa inhibitor fondaparinux was evaluated in the large outcome study OASIS 5 (Organisation to Assess Strategies for Ischemic Syndromes 5).”8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


