CARDIOHELP System is one of the first fully portable emergency life support systems for patients suffering cardiogenic shock. It measures 31 × 25 × 42 cm and weighs 11.5 kg with an ability to generate up to 6 l/minute flow with peripheral or central arteriovenous cannulation.
The National Registry of Cardiopulmonary Support for Emergency Applications details the main indications for ECPS (see Table 16.2). It reflects the large experience of using ECPS in the operating theatre and cardiology catheter labs. In this database, 63% of all patients died while on a ECPS system. Ten percent of patients lived for less than 30 days while 25% survived for more than 30 days. Un-witnessed cardiac arrest resulted in a high mortality even after resorting to ECPS.
| Indications | Percentage of patients (%) |
|---|---|
| Cardiogenic arrest post-cardiotomy | 55 |
| Cardiogenic shock | 9 |
| Cardiogenic shock post-cardiotomy | 18 |
| Hypothermia | 5 |
| Pulmonary insufficiency | 6 |
| Others | 7 |
Though the success of survival in witnessed cardiac arrest patients supported with ECPS was better than the un-witnessed group, mortality was still over 70%. Patients who survived had more therapeutic procedures undertaken than the non-survivors, implying the importance of complete correction of precipitating medical factors for a successful outcome. Though severely compromised patients can be resuscitated effectively for a period of up to 6 hours with ECPS, further therapeutic or diagnostic steps need to be undertaken in order to save the patient’s life. While ECPS is not a therapy by itself, it has been proven to buy time, potentially allowing for the correction of underlying disease processes. New heparin-bonded circuitry avoids the need for full-dose heparin, thus allowing ECPS to be used in patients with acute hemorrhage or other contraindications for extracorporeal circulation.
Definitive criteria for defining the patients who will benefit most from treatment with ECPS are still lacking, and future research should be directed to provide more information regarding this issue.
Concept of ECMO
Similar to the portable ECPS, the ECMO machine was initially developed from the concept of CPB and allows some degree of portability compared to the CPB machine. ECMO is increasingly used to provide cardiopulmonary or singular pulmonary support for patients with acute severe respiratory failure or cardiac failure. Briefly, the options are veno-arterial (VA) ECMO and veno-venous (VV) ECMO. In VA ECMO, blood is returned to the arterial system and in VV ECMO the blood is returned to the venous system. In VV ECMO, no cardiac support is provided. Figure 14.2 in Chapter 14 provides an overview of the different cannulation sites for VV and VA ECMO.
Criteria for the initiation of ECMO include acute severe cardiac or pulmonary failure that is potentially reversible and unresponsive to conventional management. The main clinical indications and contraindications for ECMO are summarized in Table 16.3.
| Indications |
|
| Contraindications |
|
ECMO set-up
During the initiation of peripheral ECMO, cannulae are inserted percutaneously. The largest cannulae that can be placed in the vessels are used in order to maximize flow and minimize pressures. ECMO required for complications of cardiac surgery can be placed directly into the appropriate chambers of the heart or great vessels, this constitutes “central ECMO.” Following heparinization and cannulation, the patient is connected to the ECMO circuit and the blood flow rate is increased until respiratory and hemodynamic stability has been achieved. Once the initial respiratory and hemodynamic goals have been achieved, the blood flow is maintained at that rate. Frequent assessment and adjustments are facilitated by continuous mixed venous oximetry to assess the adequacy of oxygen delivery. If needed, ultrafiltration can be added to the ECMO circuit to aid diuresis. For patients with respiratory failure, ECMO weaning can be started when there are improvements in radiographic appearance, pulmonary compliance, and arterial oxyhemoglobin saturation. For patients with cardiac failure, improving left ventricular output indicates that the patient may be weaned off ECMO support.
The institution of ECMO carries significant complications, which include neurological injury (subarachnoid hemorrhage, ischemic watershed infarctions, hypoxic-ischemic encephalopathy, unexplained coma, and brain death), fatal sepsis, bleeding (pulmonary hemorrhage, cannulation sites), systemic thrombo-embolism (including cardiac thrombosis from blood stasis when left ventricular output is not maintained), and anticoagulation-associated issues like heparin-induced thrombocytopenia (HIT). In addition there can be cannulation-related complications, which include vessel perforation with hemorrhage, arterial dissection, distal ischemia, and incorrect location (venous cannula within the artery). At < 5% these complications are rare.
The indications for ECMO usage are definitely on the rise. In certain settings, ECMO has been shown to provide a better or equivalent alternative to CPB, such as in re-warming from accidental hypothermia or after lung transplantation.
CPB in management of acute respiratory failure
Institution of urgent CPB is of value in patients with sustained respiratory arrest or obstruction in whom endotracheal intubation is not possible. This approach can be life- saving in young patients with treatable pathology such as mediastinal lymphadenopathy due to hematological malignancies causing superior vena cava and tracheal obstruction. Mediastinal tumors can compress major airways to such an extent that the occurrence of even mild supraglottic edema can result in complete airway obstruction. This may occur following minimal handling of the airway during attempted endotracheal intubation, or following upper respiratory tract infections. Initiation of femoro-femoral CPB is the only safe interim procedure prior to controlled tracheotomy to secure an airway. This approach provides a safe solution for airway control when intubation or a surgically created airway is either unsuccessful or too hazardous to attempt.
Management of acute pulmonary embolism
In the treatment of acute pulmonary embolism (PE), medical thrombolytic therapy is often the first-line therapy as this condition can be treated effectively and safely with thrombolytic agents, delivered either intravenously or via a pulmonary artery catheter locally. In non-surgically treated PE, emergency cardiopulmonary support with CPB in massive pulmonary embolism can be helpful in increasing the efficiency of thrombolytic agents by establishing circulation. A few instances where institution of percutaneous CPB in patients with acute pulmonary embolism was lifesaving have been reported. Pulmonary embolectomy for pulmonary thromboembolism (PE) is currently rarely indicated and only reserved as a last resort for critically ill patients with hemodynamic instability, or for those who are either not candidates for or have failed thrombolysis. When emergency pulmonary embolectomy is indicated, CPB can be useful to allow immediate resuscitation and stabilization of cardiopulmonary function prior to surgery, especially when cardiogenic shock is evident. Pulmonary embolectomy can also be achieved by pulmonary arteriotomy and retrograde flushing of the pulmonary circulation via the pulmonary veins after establishment of CPB. Numerous small-sized retrospective studies have recently reported low mortality for embolectomy when performed early and in a selected group of patients. Therefore, the criteria for surgical embolectomy could be extended from strictly rescue therapy to include hemodynamically stable patients with cardiopulmonary dysfunction.
CPB in resection of tumors
The commonest indication outside cardiac surgery to resort to CPB is the excision of a liver or renal malignancy growing into the inferior vena cava (IVC) and occasionally into the right atrium. Selective cannulation and snaring of the venae cavae, along with a generous right atriotomy after establishing CPB helps the surgeon to extract tumors extending into the inferior vena cava and right atrium under direct vision (see Figure 16.2). With tumors extending into the right atrium, or in exceptional circumstances even into the pulmonary artery, complete excision of the tumor might require deep hypothermic circulatory arrest (DHCA).
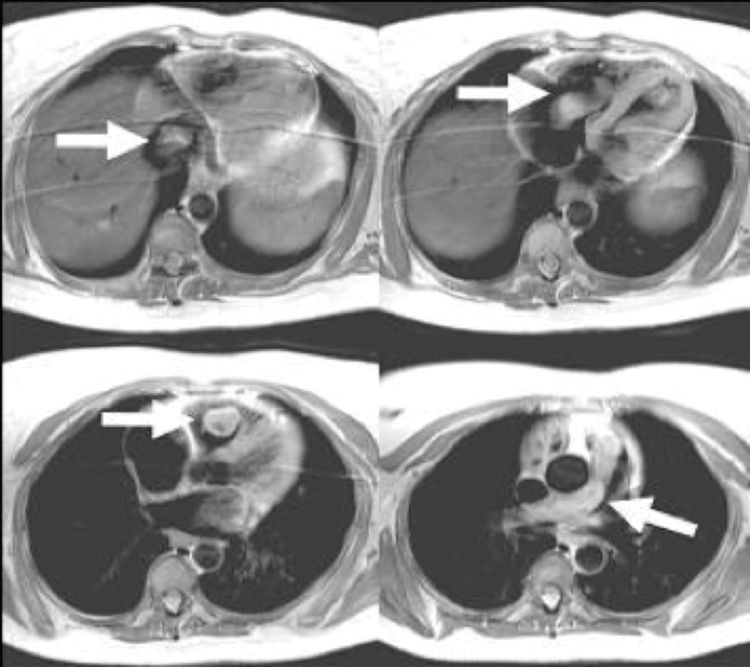
Cardiac-gated MRI scan demonstrating uterine benign leiomyoma extending from pelvis through the inferior vena cava into the right atrium, right ventricle, and subsequently into the main pulmonary artery
CPB permits maintenance of systemic perfusion at a low pressure, cessation of pulmonary artery inflow into the lungs, and, if required, drainage of the whole circulating volume into the venous reservoir, thereby allowing total circulatory arrest. The risk of stroke and other neurological complications is minimal if DHCA is employed and does not exceed 30 minutes. CPB thus enables safe resection of vascular tumors and tumors occupying anatomical locations which are difficult to access. The risk of hemorrhage and organ damage is reduced by lowering the systemic pressure on CPB, cooling down the patient, and, if required, stopping the circulation completely for a finite period of time to allow surgical dissection in a bloodless field. A recent retrospective study of 37 patients over 16 years who underwent extended resection for tumors invading the IVC with CPB with or without DHCA had low morbidity and mortality as well as acceptable survival rates, particularly if complete resection of the tumor could be achieved. Diffuse mediastinal tumors or those infiltrating the heart and great vessels are best excised after institution of CPB. Though infiltration of major vascular structures of the mediastinum can be a contraindication for attempting curative resection of advanced lung cancer, there are studies that have shown a survival benefit when performed in selected patients with advanced T4 lung tumors assisted by CPB.
CPB in other elective procedures
Resection or decompression of complex arteriovenous malformations of the retroperitoneum, mediastinum, limbs, and brain using endovascular embolization with, or without, open surgical techniques will benefit from low flow CPB or DHCA; CPB and DHCA have been of particular value in converting otherwise inoperable tumors or vascular malformations of the brain or spinal cord to those amenable to a relatively safe surgical procedure.
Profound hypothermia, circulatory arrest, and exsanguination is a common approach in certain high-risk neurosurgical interventions to remove intracranial aneurysms, glomus jugulare tumors, and hemangioblastoma of the brain. In such instances, DHCA provides a bloodless surgical field and protection of the brain, which make precise clipping of the vascular malformation possible. The disadvantages of this technique include cardiac distension and arrhythmia during CPB, hemorrhage from systemic anticoagulation, and central nervous system injury due to inadequate cerebral protection.
CPB in non-cardiac transplantation
Single and sequential double lung transplantation
CPB has been frequently used for single and double lung transplantation. Most commonly, however, the decision to establish partial or complete CPB is made after hemodynamic assessment of the patient following occlusion of the pulmonary artery during surgery. Criteria for the establishment of CPB include a mean pulmonary artery pressure of more than 50 mmHg, hypoxia, hypercapnea, or hemodynamic instability. Prior to surgery it is also possible to get an indication of the need for CPB support by eliminating ventilation to the operative lung. If the non-operative lung is ineffective for maintaining ventilation on its own, the patient is unlikely to tolerate the period of lung isolation during explant and implant of the operative lung and CPB will be required.
As yet, there are no reliable preoperative predictors for the need for CPB in lung transplantation. However, in a study involving 109 lung transplant recipients, preoperative right ventricular ejection fraction of < 40%, 6-minute walk test result of less than 250 m, and drop in arterial oxygen saturation on exercise to < 94% on room air were positive predictive factors for resorting to CPB.
Some transplant centers have been reluctant to use CPB during lung transplantation due to potential side effects, including hemorrhage and triggering of a systemic inflammatory response syndrome (SIRS), leading to sequestration of neutrophils and platelets in the pulmonary capillary bed, endothelial damage, increased capillary permeability, and subsequent pulmonary edema. There are numerous retrospective studies demonstrating higher short-term mortality and primary allograft dysfunction with the usage of CPB compared to without and, moreover, citing worse outcomes with unplanned CPB than planned CPB. This is contradicted by the findings of Hlozek et al. from the Cleveland Clinic, involving 74 patients over 4 years. Comparing patients who had their lung transplant with or without CPB, it failed to demonstrate any significant difference in the short- or long-term outcome between the groups. At the current time, there are numerous comparative studies assessing the benefits of CPB versus ECMO for lung transplantation which demonstrate that there are no significant differences in 30-day, 90-day or 1-year mortality, although the use of ECMO generally results in less bleeding and requires fewer blood product transfusions.
Liver transplantation
Occasionally extracorporeal circulation is used to assist liver transplantation (LTX). Cardiopulmonary bypass in LTX is indicated for hemodynamic rescue and, at some centers, serves as the hemodynamic support during liver implantation. In general terms, extracorporeal circulation provides a means of decompressing the hepato-portal circulation and reducing the risk of bleeding when operating on patients with portal hypertension. It also reduces the risk of post-transplantation renal failure and of intestinal venous congestion with subsequent hepatic dysfunction. The femoral vein is cannulated for venous return using a standard short venous cannula. The venous blood thus drained is passed through a centrifugal pump to be returned to the systemic venous circulation by cannulae inserted into the internal jugular or subclavian vein. Heparin-bonded circuits are preferred to avoid full systemic heparinization.
Systemic venous return is often impaired by surgical manipulation during both excision of the native liver and implantation of the transplant organ. Employing an extracorporeal perfusion technique allows the portal circulation to be decompressed as well as systemic venous return to be maintained at levels adequate to maintain cardiac output. Furthermore, it allows extravasated blood to be salvaged and returned to the circulation. If portal hypertension persists despite inferior vena cava drainage via the femoral vein, an extravenous drainage cannula can be inserted directly into the portal vein. It should be noted that the circuit described here is not a CPB circuit as it does not include an oxygenator and there is no arterial cannulation because the heart maintains the cardiac output.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


