New-onset left bundle branch block (LBBB) is a specific concern of transcutaneous aortic valve implantation (TAVI) given its estimated incidence ranging from 5% to 65%. This high rate of occurrence is dependent on the type of device used (size and shape), implantation methods, and patient co-morbidities. The appearance of an LBBB after TAVI reflects a very proximal lesion of the left bundle branch as it exits the bundle of His. At times transient, its persistence can lead to permanent pacemaker implantation in 15% to 20% of cases, most often for high-degree atrioventricular block. The management of LBBB after TAVI is currently not defined by international societies resulting in individual centers developing their own management strategy. The potential consequences of LBBB are dysrhythmias (atrioventricular block, syncope, and sudden death) and functional (heart failure) complications. Prompt postprocedural recognition and management (permanent pacemaker implantation) of patients prevents the occurrence of potential complications and may constitute the preferred approach in this frail and elderly population despite additional costs and complications of cardiac pacing. Moreover, the expansion of future indications for TAVI necessitates better identification of the predictive factors for the development of LBBB. Indeed, long-term right ventricular pacing may potentially increase the risk of developing heart failure in this population. In conclusion, it is thus imperative to not only develop new aortic prostheses with a less-deleterious impact on the conduction system but also to prescribe appropriate pacing modes in this frail population.
Severe symptomatic aortic stenosis is a common and serious disease. Surgical aortic valve replacement (SAVR) is the gold standard for the treatment of operable patients. Transcutaneous aortic valve implantation (TAVI) was developed as an alternative for inoperable patients or patients at high surgical risk. In the absence of effective medical management, TAVI in this population has resulted in an improved prognosis because mortality in this population is similar to SAVR at 30 days and 1 year. However, the proximity of the conduction system located in the septum explains the higher incidence of conduction disorders after TAVI. The management of persistent complete atrioventricular block (AVB) is straightforward and requires permanent pacemaker implantation (PPI). Conversely, the management of left bundle branch block (LBBB) in this patient population is not yet clearly established. This sets the stage for the occurrence of 2 types of complications: (1) complete AVB, syncope, and sudden death (SCD); (2) heart failure (HF) development. The present literature review assesses the epidemiology and pathophysiology of LBBB after TAVI and its clinical and prognostic consequences, in addition to proposing management options.
Epidemiology
New-onset LBBB is one of the most common complications after TAVI despite its highly variable incidence, estimated at 5% to 65% depending on the study. This considerable heterogeneity can be explained by the morphology of the 2 main types of prostheses used (Medtronic CoreValve and Edwards SAPIEN family [XT and Sapien 3]). LBBB after TAVI is 3 times more common with the CoreValve system (CVS) compared to Edwards system (30% to 60% vs. 6% to 12% ; Figure 1 ). This higher rate of LBBB with CVS is explained by the design of the prostheses and the greater depth in the left ventricular (LV) outflow tract. Modern technology may decrease the rate of LBBB after TAVI as shown with Corevalve EvolutR. However, the Edwards Sapien 3, recently developed, is associated with more LBBB than the previous system (Edwards Sapien XT [24% vs. 7.1%, p <0.001]) because of the new design: higher radial strength and outer skirt. In addition, the learning curve for this technique is steep and could account for some variation. It is important to emphasize that the incidence of LBBB after TAVI remains much higher than that observed after SAVR (4%).

The onset and disappearance of LBBB are poorly described. LBBB can occur unpredictably, that is, during preballoon dilatation of the aortic annulus such as in the CVS where it is associated with almost half of periprocedural LBBBs. Most conduction disturbances (90%) occur during the first week after implantation. New-onset LBBB can be transient and hence disappear within the first few days in 19% to 34% of patients but mostly persists at 30 days (62%). It can also be permanent and persist in 2 of 3 patients at 1 year and can also appear up to 1 year after procedure in 0.8% of patients. Finally, spontaneous recovery from LBBB is much less frequent when using the CVS compared to Edwards system (39% vs. 9.5%).
Pathophysiology of TAVI-Induced Electrical Disturbances
The onset of LBBB after TAVI indicates a very proximal lesion of the left bundle branch at the immediate exit of the bundle of His because LBBB pattern in electrocardiography after TAVI is reproducible and “pure.” The occurrence of this lesion stems from the anatomic relation between the implantation site of the aortic prosthesis and the conduction pathways but also as a result of the implantation techniques and the morphology of the prostheses used.
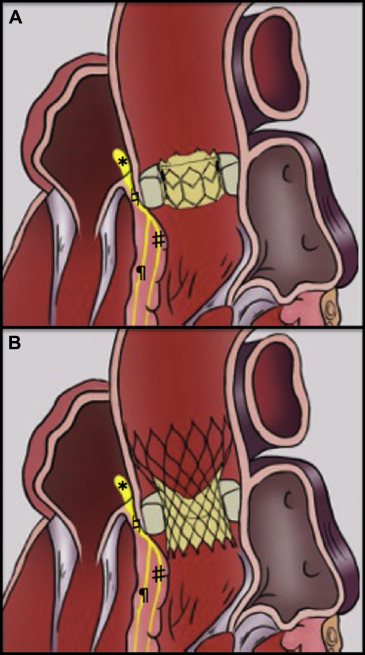
From an anatomic standpoint, the aortic annulus is in close proximity to the atrioventricular (AV) nodal-Hisian conduction systems, especially the left bundle branch. Indeed, the aortic bioprosthesis and its structure are supported by the aortic annulus and the native calcified aortic valve. These 2 structures are dilated and crushed by the implanted valve at the level of the aortoventricular junction and the subaortic annulus ( Figure 2 ). As a result of its anatomic location, that is, a few millimeters below the annulus, the left bundle branch is the most exposed thus explaining the high incidence of LBBB after TAVI. It thus appears obvious that the implantation depth of the valve is a critical factor in the development of LBBB.

The CVS is 5 times more prone to the implantation of a pacemaker at 1 year (25.8% vs. 6.5% for the Edwards Sapien Valve). Its larger size and in particular its greater depth in the LV outflow tract, its more rigid nitinol alloy, its self-expansion, and its ovoid form likely exert greater pressure on the conduction pathways. The kinetics of appearance and persistence of an LBBB after a TAVI procedure are variable and stem from different pathophysiological mechanisms. Indeed, the early onset of an LBBB reflects an initial lesion of the left branch either as a result of edema (transient LBBB) or by direct injury (permanent LBBB). This early damage may also be simply due to balloon predilatation. Finally, the onset of LBBB can be delayed possibly because of continued calcification of the conduction pathways. Finally, the continued expansion of the prosthesis may also explain the late onset of LBBB.
Pathophysiology of TAVI-Induced Electrical Disturbances
The onset of LBBB after TAVI indicates a very proximal lesion of the left bundle branch at the immediate exit of the bundle of His because LBBB pattern in electrocardiography after TAVI is reproducible and “pure.” The occurrence of this lesion stems from the anatomic relation between the implantation site of the aortic prosthesis and the conduction pathways but also as a result of the implantation techniques and the morphology of the prostheses used.
From an anatomic standpoint, the aortic annulus is in close proximity to the atrioventricular (AV) nodal-Hisian conduction systems, especially the left bundle branch. Indeed, the aortic bioprosthesis and its structure are supported by the aortic annulus and the native calcified aortic valve. These 2 structures are dilated and crushed by the implanted valve at the level of the aortoventricular junction and the subaortic annulus ( Figure 2 ). As a result of its anatomic location, that is, a few millimeters below the annulus, the left bundle branch is the most exposed thus explaining the high incidence of LBBB after TAVI. It thus appears obvious that the implantation depth of the valve is a critical factor in the development of LBBB.
The CVS is 5 times more prone to the implantation of a pacemaker at 1 year (25.8% vs. 6.5% for the Edwards Sapien Valve). Its larger size and in particular its greater depth in the LV outflow tract, its more rigid nitinol alloy, its self-expansion, and its ovoid form likely exert greater pressure on the conduction pathways. The kinetics of appearance and persistence of an LBBB after a TAVI procedure are variable and stem from different pathophysiological mechanisms. Indeed, the early onset of an LBBB reflects an initial lesion of the left branch either as a result of edema (transient LBBB) or by direct injury (permanent LBBB). This early damage may also be simply due to balloon predilatation. Finally, the onset of LBBB can be delayed possibly because of continued calcification of the conduction pathways. Finally, the continued expansion of the prosthesis may also explain the late onset of LBBB.
Technical and Clinical Predictive Factors for New-Onset LBBB After TAVI
Various predictors of the occurrence of LBBB after TAVI are resumed in Table 1 . All have as consequence a direct lesion to the proximal left bundle branch as a result of mechanical trauma, by the use of either a predilatation balloon or the prosthesis.
| Study | LBBB/Study pop. | OR (IC 95%) | p-value | |
|---|---|---|---|---|
| Medtronic CoreValve System | Aktug et al. | 40/139 | 2.639 (1.314-5.813) | 0.007 |
| Franzoni et al. | 63/238 | 7.2 (2.9-17.4) | 0.001 | |
| Houthuizen et al. | 233/679 | 8.51 (5.53-13.11) | 0.001 | |
| Schymik et al. | 197/634 | 2.5 (1.668-3.799) | 0.001 | |
| Men | Franzoni et al. | 63/238 | 3.96 (1.12-14.06) | 0.033 |
| Depth of the prosthesis in the outflow tract | Boerlage et al. | 47/121 | 1.3 (1.09-1.57) | 0.004 |
| Van der Boon et al. | 185/549 | 1.16 (1.10-1.24) | 0.001 | |
| Aktug et al. | 40/139 | 1.185 (1.064-1.320) | 0.002 | |
| Urena et al. | 61/202 | 1.37 (1.06-1.77) | 0.017 | |
| Piazza et al. | 34/91 | 1.2 (1.32-3.17) | 0.001 | |
| Katsanos et al. | 15/94 | 1.401 (1.06-1.770) | 0.010 | |
| Prior myocardial infarction | Piazza et al. | 34/91 | 3.48 (1.00-12.05) | 0.049 |
| Right bundle branch block | Piazza et al. | 34/91 | 15 (1.43-157.9) | 0.024 |
| 26 mm-diameter prosthesis (CoreValve) | Boerlage et al. | 47/121 | 4.1 (1.32-12.34) | 0.01 |
| Overexpansion of the prosthesis > 15% | Katsanos et al. | 15/94 | 5.277 (1.398-19.919) | 0.014 |
| Previous coronary bypass | Schymik et al. | 197/634 | 1.64 (1.003-2.688) | 0.136 |
| Prolonged baseline QRS duration | Urena et al. | 61/202 | 1.24 (1.01-1.50) | 0.037 |
| Diabetes mellitus | Houthuizen et al. | 233/679 | 1.52 (1.01-2.29) | 0.04 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


