SEP monitoring has been used for monitoring spinal cord function for many years, and more recently has been used to monitor other parts of the neuroaxis as well. It is well suited to NIOM as it is typically “invisible” to the surgeon, that is, it causes no significant disruption of surgical technique or movement in the operative field. The responses are relatively robust and consist of various components that serve as markers of the integrity of various parts of the somatosensory pathways. Essential aspects of SEP monitoring are described here.
Anatomy and Physiology
SEP monitoring is performed with the stimulation of large peripheral nerves in the extremities. In the upper limbs, SEP are obtained with stimulation of the median or ulnar nerves, and in the lower extremities, they are obtained with stimulation of the tibial or peroneal nerves. Electrical stimulation of the peripheral nerves results in the activation of sensory and motor pathways, with the largest fibers being activated preferentially. The activation of the sensory fibers produces an orthodromic volley of nerve fiber activation that produces the various components of the SEP waveform. These components reflect the activation of the dorsal column pathways.
Stimulation of the median or ulnar nerves results in the activation of sensory fibers that relay up to the respective dorsal root ganglia. The axons from the dorsal root ganglia travel through the dorsal roots of the spinal nerves into the dorsal column pathway in the spinal cord. They ascend to synapse with second-order neurons in the nucleus cuneatus in the medulla. The second-order neurons decussate in the medulla to ascend in the contralateral medial lemniscus to the ventroposterior lateral (VPL) nucleus of the thalamus where they synapse with third-order neurons. These third-order neurons then ascend in the thalamocortical radiation to the hand area of the sensory homunculus of the primary sensory cortex on the lateral aspect of the postcentral gyrus. Tibial and peroneal nerve stimulation results in the activation of the dorsal column pathway corresponding to these nerves. This pathway is very similar to that described for the median and ulnar nerves, except that instead of the first-order neurons relaying on the nucleus cuneatus, they synapse in the nucleus gracilis. Additionally, the thalamocortical fibers relay in the vertex region of the somatosensory cortex corresponding to the leg and foot area of the sensory homunculus.
The SEP waveform reflects transmission of a volley of depolarization as it ascends the dorsal column pathway. The waveforms are similar to those recorded in the clinical laboratory, as are the montages. Each waveform receives contributions from several neuroanatomic structures, and various neuroanatomic structures contribute to several waveforms. Despite this, it is useful to think of various components of the SEP waveforms as arising from general areas of the neuroaxis. This helps identify potential sites of compromise during surgery. The common waveforms recorded after stimulation of an upper extremity nerve (median or ulnar) are the Erb’s point (EP, N9), cervical (N13), subcortical (P14 and N18), and cortical (N20) potentials. After lower extremity nerve (tibial) stimulation, the commonly recorded waveforms are the popliteal fossa (PF), lumbar (LP), subcortical (P31 and N34), and cortical (P37) potentials. The generators most often associated with each of these waveforms are presented in Table 16.1 (1).
SEP Waveforms, Generators, and Montages
Label | Generator |
Upper extremity (median and ulnar nerve) | |
EP (N9) N13 P14 N18 N20 | Brachial plexus Cervical spinal cord Nucleus cuneatus/medial lemniscus Thalamus Somatosensory cortex |
Lower extremity (tibial nerve) | |
PF LP P31 N34 P37 | Tibial nerve in popliteal fossa Cauda equina/lumbar segments of spinal cord Nucleus gracilis/medial lemniscus Thalamus Somatosensory cortex |
EP, Erb’s point; PF, popliteal fossa; LP, lumbar potential.
Since SEP monitoring is used most often to monitor the integrity of the spinal cord, it is important to understand the vascular supply of the spinal cord and how it may impact SEP NIOM. The spinal cord receives blood supply through a network of one anterior and two posterior spinal arteries. The single anterior spinal artery extends from the cervicomedullary junction to the filum terminale. It is formed by branches from both vertebral arteries and receives perforators from the aorta. In the embryo, radicular arteries arise from the aorta at each segmental level and divide into anterior and posterior branches. The anterior branches supply the anterior spinal artery. At the time of birth, only two to eight of these segmental radicular arteries remain; two to three arise from cervical levels and one to three from the thoracic levels (2). A large segmental radicular artery arising most often between T9 and T12, known as the artery of Adamkiewicz, supplies the caudal part of the spinal cord. The actual number of radicular branches that supply the spinal cord is highly variable in adults. The midthoracic spinal cord is the least well supplied, and consequently is most prone to ischemic injury (3). The anterior spinal artery supplies the anterior two-thirds of the spinal cord, which includes the lateral and anterior corticospinal tracts.
The paired posterior spinal arteries also arise from the vertebral arteries. These arteries receive posterior branches of the radicular arteries. While there is an extensive anastomotic network between the paired posterior spinal arteries, such an anastomotic network does not exist between the posterior spinal arteries and the anterior spinal artery (2). The posterior spinal arteries supply the posterior one-third of the spinal cord, including the dorsal column pathways.
Given the vascular distributions of the anterior and posterior spinal artery systems, selective injury to the anterior spinal artery can result in infarction of the anterior two-thirds of the spinal cord, affecting mostly motor pathways and sparing the dorsal column pathways. During NIOM, this would be manifest as preserved SEP but possible change in MEP (discussed later). Conversely, selective injury of the posterior spinal artery system can result in infarction of the posterior one-third of the spinal cord, affecting mostly dorsal column pathways and sparing the motor pathways. This type of injury is much less common than selective injury to the anterior spinal artery. Understanding the vascular anatomy of the spinal cord helps in explaining the rationale for SEP and MEP monitoring.
Methodology
The discussion of SEP methodology will involve stimulating and recording techniques. Differentiating artifact-induced change in SEP signals from surgical changes is one of the main functions of the interpreting physician.
Stimulating Techniques
SEP used in NIOM are obtained after stimulating large peripheral nerves. This section will review the types of electrodes that can be used for stimulating peripheral nerves and stimulation parameters.
Stimulating Electrodes
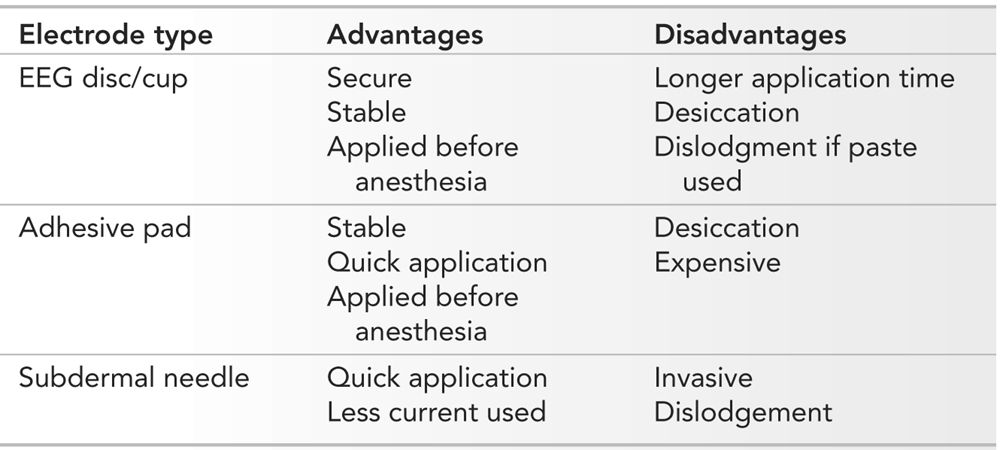
The requirements for a stimulating electrode used for NIOM are that it must be easy and quick to apply, remain stable during patient movement and positioning, and provide consistent stimulation of the peripheral nerve. There are many different types of electrodes that can be used; however, three main types are used most frequently. These are EEG discs, adhesive pads, and subdermal needles (Fig. 16.1). Each has advantages and disadvantages, and their selection will depend on the type of surgery to be monitored. EEG disc electrodes are stable and are secure when applied with collodion. The cup is filled with a conductive gel. They can be applied before the patient is anesthetized. The major disadvantage of these electrodes is the time required to apply them, especially when collodion is used. Collodion also mandates adequate ventilation as the fumes may be bothersome to both staff and patients. During long surgeries, the conductive gel may dry, hindering adequate stimulation. If paste is used instead of collodion, the electrodes may become dislodged with patient positioning.
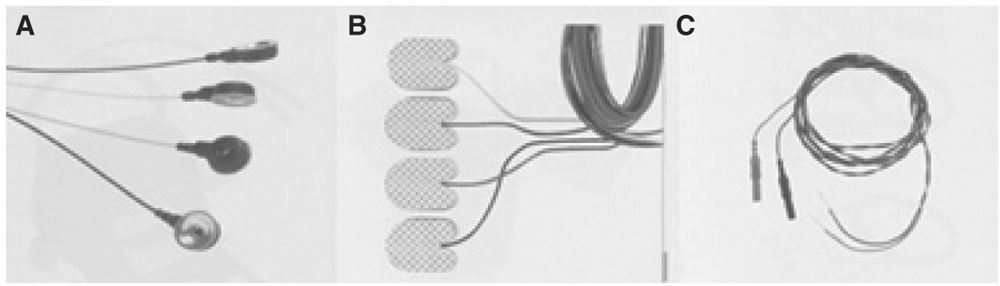
Figure 16.1: Illustration of EEG cup (A), adhesive pads (B), and subdermal needle (C) electrodes used for stimulating peripheral nerves.
Adhesive pad electrodes are easy and quick to apply. They are available in different sizes and are stable after application. They are not easily dislodged with patient movement and positioning. Like EEG disc electrodes, they can be applied prior to the patient being anesthetized. The adhesive pad electrodes come prepackaged with conductive gel; however, during long surgeries, the gel may dry, impairing proper stimulation. Another disadvantage is their price.
Experienced technologists can apply subdermal needle electrodes very quickly. They require less stimulation intensity. If accidentally removed, they can be reapplied more easily than EEG disc electrodes. The major disadvantage of subdermal needle electrodes is that they are invasive and must be applied after the patient has been anesthetized. Though they can be secured, they can also be pulled out more easily during patient movement and positioning. A summary of the advantages and disadvantages of the various electrodes is presented in Table 16.2.
Advantages and Disadvantages of Various Types of Stimulating Electrodes
Stimulation Parameters
Suggested parameters for stimulating peripheral nerves to obtain SEP for NIOM have been recommended by the ACNS (4). A constant-current stimulator is recommended as it compensates for changes in tissue impedance. A rectangular pulse between 100 and 300 μs duration is used. An intensity of stimulus is used that provokes a visible muscle twitch. It is generally a good idea to increase the intensity a little more than that required for a twitch to ensure adequate stimulation. Usually a stimulus intensity of 30 to 40 mA will be needed; however, if the nerve is diseased, higher intensities may be required. When needle electrodes are used, considerably less stimulus intensity is needed to produce adequate stimulation. Even with high stimulation intensities, tissue damage does not occur. The recommended stimulation rate is 2 to 8 per second (5.7 is common). As with all types of evoked potentials, an integer of 60 should be avoided to maximize cancellation of 60-Hz electrical noise. The electrode impedance is kept below 5,000 Ω. Unilateral stimulation is preferred as bilateral stimulation can mask a unilateral change in potentials. Modern NIOM equipment is capable of independently stimulating both sides in an interleaved manner so that both responses are obtained simultaneously.
Recording Techniques
Various electrodes, sites, and montages can be used to record SEP waveforms. This section will review these as well as recording parameters.
Recording Electrodes
EEG disc and subdermal needle electrodes can also be used for recording SEP. Additionally, corkscrew and epidural electrodes can also be used (Fig. 16.2). EEG disc electrodes have many of the same advantages and disadvantages as when they are used for stimulation. Subdermal needles are often used for recording as well. In addition to the advantages and disadvantages mentioned already, they record large amplitude responses.

Figure 16.2: Illustration of corkscrew (A) and epidural (B) electrodes.
Corkscrew electrodes have the advantage of being very secure and do not move with patient movement and positioning. They are also quick to apply. However, their invasive nature mandates that they be applied after the patient has been anesthetized. Although they can be used for recording SEP, they are used more often as stimulating electrodes for MEP.
Epidural electrodes, as the name implies, are placed in the epidural space. These electrodes record directly from the surface of the spinal cord, and consequently, have robust and large signals that require very few repetitions to produce reproducible waveforms. Because of their quick reproducibility, feedback to the surgeon is quicker with epidural electrodes. Epidural responses are also insensitive to anesthetics. The disadvantage of these electrodes is that they are invasive and must be placed and anchored by the surgeon. Movement of the electrode, which is not uncommon, can significantly change the morphology of the response. These electrodes also cannot be used in the cervical region and when an anterior approach is used. Other types of invasive electrodes, such as needle electrodes that are placed in the ligamentum flavum, have many of the advantages and disadvantages of epidural electrodes. All these electrodes in the past were also used to stimulate the spinal cord and produce what were thought to be MEP from lower extremity muscles. Since spinal cord–induced MEP are now thought to be mostly mediated by sensory pathways and with the availability of transcranial MEP, epidural, and other similar electrodes are now seldom used for stimulation. A summary of the advantages and disadvantages of various recording electrodes is presented in Table 16.3.
Advantages and Disadvantages of Various Types of Recording Electrodes

Recording Sites and Waveforms
SEP waveforms are recorded at various points along the neuroaxis. This provides redundancy in recording the responses, as well as provides better localization of problems when waveforms are lost. Though recorded from mostly different sites, SEP waveforms from the upper and lower extremities are largely congruous. Each recording site is particularly useful for recording a particular SEP waveform.
Peripheral responses, the EP and PF responses, as their names imply, are recording from the Erb’s point and popliteal fossa. These responses identify adequacy of stimulation of the peripheral nerve. The responses are of high amplitude and easily reproducible. Since they are peripheral responses, they do not reflect problems with the central nervous system (CNS).
Cervical (N13) and lumbar (LP) responses represent the first CNS volley of the SEP obtained after stimulation of the upper and lower extremities, respectively. Since electrodes needed to record these responses are placed on the cervical and thoracolumbar spine, they are often in the surgical field and cannot be used.
Since subcortical potentials (P13/14 and N18 after upper extremity stimulation; P31 and N34 after lower extremity stimulation) are far-field potentials, they are recorded from the same set of electrodes. Typically, the active electrode is placed on the scalp and the reference on the mastoid, ear, or contralateral EP. Subcortical potentials are relatively insensitive to inhalational anesthetics, making them useful in determining when a change in cortical potentials is due to anesthetics versus surgical intervention. Their biggest limitation is their small amplitude. This makes them difficult and time consuming to record. They are recorded best with muscle relaxants as that diminishes muscle activity, increasing the signal-to-noise ratio (SNR). Unfortunately, excessive muscle relaxation precludes the use of MEP. False-positive changes in subcortical potentials are not uncommon and must be recognized in order to avoid unnecessary delays in surgery.
Cortical potentials (N20 after upper limb and P37 after lower limb stimulation) are near-field potentials. The optimal recording location for the N20 potential is between the C3/4 and P3/4 electrode (referred to as the CP3/4 electrode), contralateral to the side of stimulation. This is referenced to the congruous contralateral electrode. The best location for recording the P37 potential is more variable. At times, it is best recorded over CPz, whereas in other patients, it is of higher amplitude over CP ipsilateral to the side of stimulation. Fz or CP contralateral to side of stimulation is used as a reference electrode. The variable topography of, particularly, the P37 may necessitate additional recording electrodes (5). Cortical potentials are usually of high amplitude and easy to reproduce. Inhalational anesthetics have a profound effect on cortical potentials. This can be an advantage (to provide an indication of anesthetic management) or a disadvantage (false positives).
Recording Montages
Montages must be designed to maximize the SNR of the SEP waveforms to allow rapid feedback to the surgeon. Peripheral, subcortical, and cortical potentials should be displayed whenever possible. Modern NIOM machines have 8 to 32 channels and allow such a display, depending on which other modalities are being monitored. The ACNS has recommended montages for upper and lower extremity SEP (4). These, along with the author’s modifications, are presented in Table 16.4.
Typical Montage Used for Upper and Lower Extremity SEP
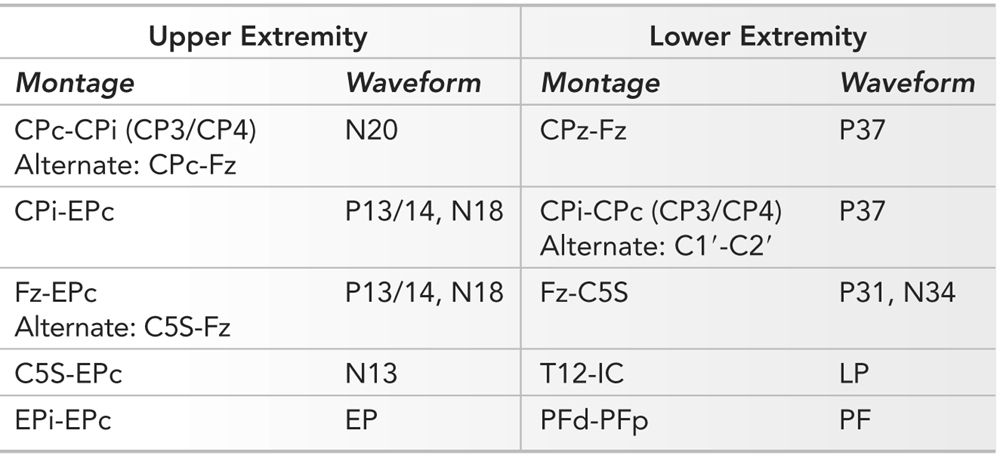
In recording upper extremity SEP, a four- to five-channel montage is usually used. The CPc-CPi (c = contra, i = ipsi) derivation is useful for recording the cortical N20 waveform. Since both electrodes are on the scalp, subcortical components cancel out. The CPi-EPc and Fz-EPc derivations both have one scalp and one nonscalp reference electrode. The scalp electrodes are distant from the activated somatosensory cortex and consequently do not record the cortical waveform. However, they do record the subcortical responses since they are referenced to an electrode (EPc), which is “silent.” The C5S-Fz derivation is used less often as it displays the N13 and P13/14 and N18 complex in a single channel. Separating the cervical (N13) from the subcortical (P13/14 and N18) becomes difficult. The C5S-EPc derivation records activity from the cervical spine (N13). The C5S electrode is often in the surgical field, so this derivation is often not used. The Epi-EPc records the potential as it passes through the brachial plexus.
Lower extremity SEP are also recorded with a four- to five-channel montage. The CPz-Fpz and CPi-CPc (or C1′-C2′) derivations record the cortical P37 potentials. In both derivations, electrodes are on the scalp; subcortical potentials are not seen as they cancel out. Two derivations are used to record the cortical waveform as its topography is variable, and if a single derivation is used, the best waveform morphology may not be seen. A more thorough approach to find the best derivation can be undertaken and is described elsewhere (5). Identifying the set of electrodes that maximizes the SNR of the P37 waveform will increase the speed with which reproducible responses can be obtained, thereby increasing speed of surgical feedback. The Fz-C5S derivation is used to record the subcortical waveforms; there is a scalp electrode removed from the activated somatosensory cortex referenced to a “silent” electrode (C5S). The C5S electrode is silent when recording lower extremity SEP. This is not the case when recording upper extremity SEP. The T12-IC (iliac crest) derivation is useful for recording the LP potential. However, as with the C5S electrode, the T12 electrode is often in the surgical field and thus often not used. The PFd-PFp records the traveling wave of depolarization as it passes in the tibial nerve under the PFd electrode. This latter derivation cannot be used if the peroneal nerve is used for stimulation.
Recording Parameters
Recording parameters have been recommended in the ACNS guidelines (4). These guidelines have suggested that 500 to 1,000 repetitions should be obtained for each upper extremity SEP and 1,000 to 3,000 repetitions for each lower extremity SEP. Others have suggested that fewer repetitions should be used if reproducible responses are seen earlier as this will increase the rapidity of feedback to the surgeon (5).
The time base for recording (time displayed on the screen) is usually set to twice the duration of the last peak of interest. Consequently, for upper extremity SEP, the time base is 30 to 100 milliseconds, while for lower extremities it is 75 to 150 milliseconds. Filters are used to minimize noise and activity that is not of interest. This enhances the SNR, and when set appropriately, filters can increase the rapidity with which reproducible waveforms are seen. A filter setting of 30 to 3,000 Hz is usual. Dropping the low-frequency filter to 1 Hz introduces excessive baseline fluctuation, making it more difficult to resolve waveforms. Increasing this filter too high (to 75 Hz) reduces the amplitude of slow components of the SEP (6).
Anesthetic Considerations
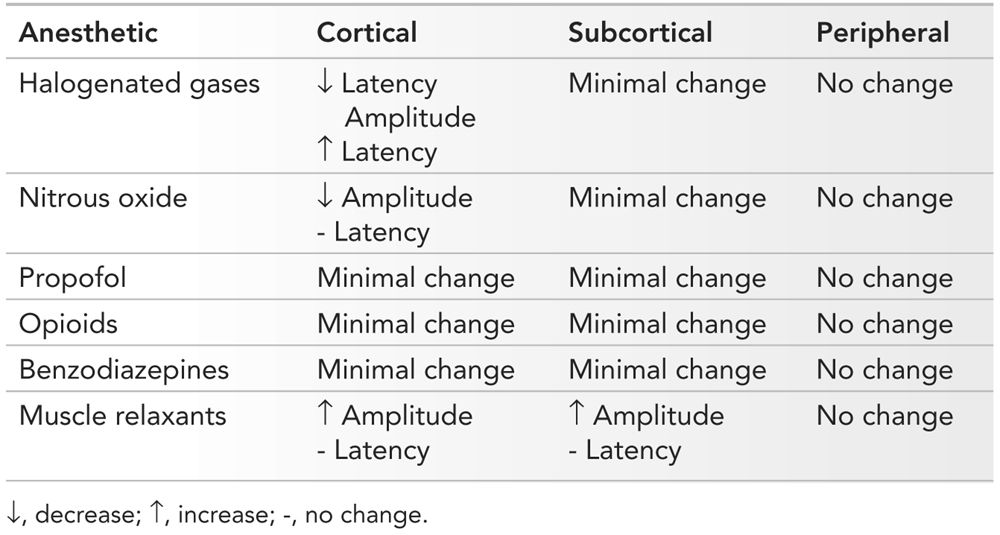
Anesthetics can have a profound effect on SEP, and these must be recognized to provide appropriate interpretation of changes (7). The higher the dose of an anesthetic, the more it depresses the SEP. Since synapses are affected most by anesthetics, those SEP waveforms produced prior to any synapses are affected least, while those occurring after several synapses are affected most. Thus, cortical responses are more sensitive to anesthetics than subcortical and peripheral responses. Anesthesiologists can use several different medications, and a summary of their effects will be presented here and in Table 16.5.
Summary of Effects of Anesthetics on SEP
Inhalational Agents
There are several types of halogenated gases, and they differ in their potency. Isoflurane, desflurane, and sevoflurane are more potent than halothane and enflurane. Generally, the more potent the agent, the greater the effect on SEP. Cortical components of the SEP are affected most by halogenated gases, and the higher the dose, the greater the effect (8). Subcortical components are affected less, and peripheral responses are unaffected (Fig. 16.3). With less than 0.5 MAC of any halogenated gas, usually subcortical waveforms can be reliably reproduced and followed in spine surgeries. It should be remembered that changes in SEP lag behind changes in end-tidal concentrations of inhalational agents. This is because there is a 15- to 20-minute lag between equilibration of lung and brain concentrations.
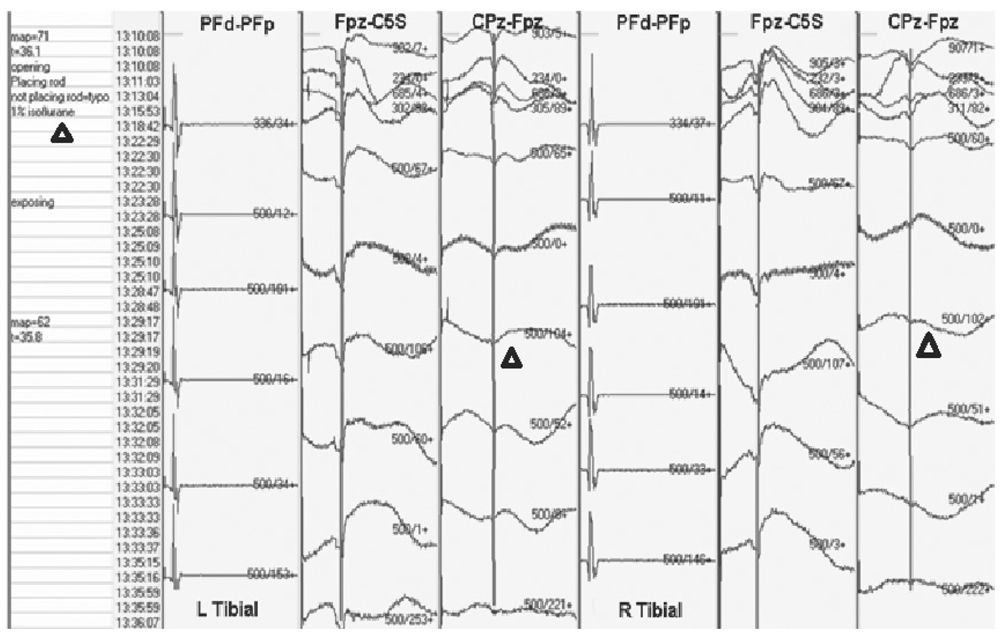
Figure 16.3: SEP recording showing loss of cortical waveforms with little effect on subcortical waveform when isoflurane dose is increased (triangles).
The effects of nitrous oxide on SEP are similar to those of halogenated gases. It causes a significant decrease in the amplitude of the cortical potentials and has less effect on subcortical potentials. An equipotent dose of nitrous oxide produces more amplitude suppression of cortical potentials than halogenated gases. If cortical potentials are necessary to record, nitrous oxide should be avoided.
Intravenous Agents
Propofol is an intravenous (IV) agent that has become very popular as part of total intravenous anesthesia (TIVA). It suppresses the cortical potentials much less than inhalational agents and only at high doses. Similarly, it causes minimal change in subcortical potentials. It has become the agent used most often if cortical potentials must be recorded and when MEP monitoring is being considered.
Fentanyl and other IV opioids are useful adjuncts to propofol in TIVA. They also cause minimal depression of the cortical and subcortical waveforms and can be used successfully with MEP monitoring. Opioids, however, cannot be used in isolation as they do not provide sufficient sedation.
Sedatives such as benzodiazepines are often used during induction and to reduce anesthetic recall. At low doses, they have little effect on cortical and subcortical potentials. However, at high doses, they can suppress cortical potentials. If benzodiazepines are used only during induction, even at high doses, they are seldom problematic during the critical portions of the surgery.
Neuromuscular Junction Blocking Agents
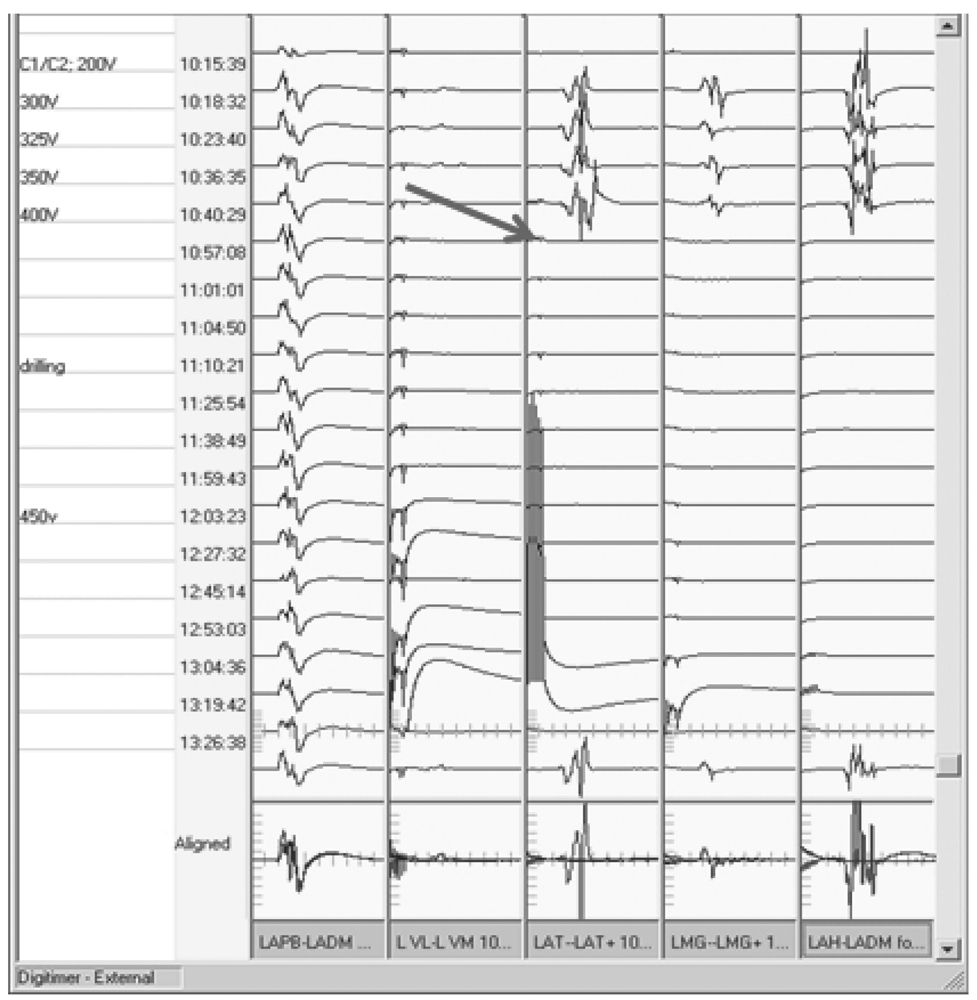
Rather than suppressing SEP waveforms, neuromuscular junction blocking agents (NMJBA) often appear to increase amplitudes of particularly the subcortical waveforms. This is because they suppress the underlying muscle activity, and thereby improve the SNR (Fig. 16.4). It should be remembered, though, that use of NMJBA can complicate MEP monitoring, as will be discussed later.
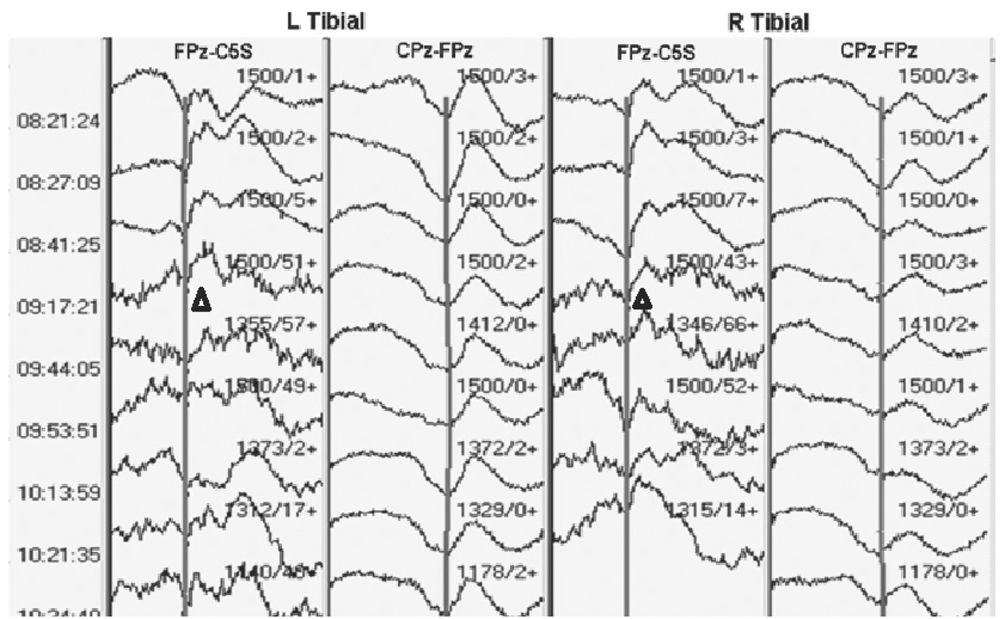
Figure 16.4: SEP recording showing worsening subcortical waveforms as muscle relaxant wears off (triangles). The muscle relaxant was given 5 minutes prior to the first tracing shown.
Mechanisms of SEP Change
There are many different reasons why SEP waveforms can change during surgery (9). Some are related directly to the surgical intervention, while others are due to changes in the physiologic milieu, and still others are due to technical issues.
Ischemia and Anoxia/Blood Pressure
There are few data on the changes in SEP induced by ischemia of the spinal cord. However, there are considerable data on the effect of ischemia on peripheral nerves. After about 20 minutes of ischemia, nerve potentials drop by 50% owing to temporal dispersion. The latency also prolongs by 1 to 2 milliseconds (10). A drop in blood pressure induces relative ischemia of the spinal cord, and increasing the blood pressure has been associated with improvement in SEP (Fig. 16.5). In one study in 9 of 11 patients in whom an alert was raised due to reduced SEP amplitude, an increase in blood pressure improved the responses (11).
Figure 16.5: SEP recording showing a decrease in amplitude of the cortical and subcortical waveforms after tibial nerve stimulation with decreasing mean arterial blood pressure (triangles), with recovery as the pressure increases (arrows).
Impairment of blood supply to the nerve being stimulated to obtain a SEP can result in loss of amplitude of the SEP. This will usually be accompanied by loss of the peripheral (PF or EP) response as well and will be limited to one extremity (Fig. 16.6). This can happen if an ace wrap is applied too tightly or if a cannula is placed in a large artery supplying the limb, making it ischemic.
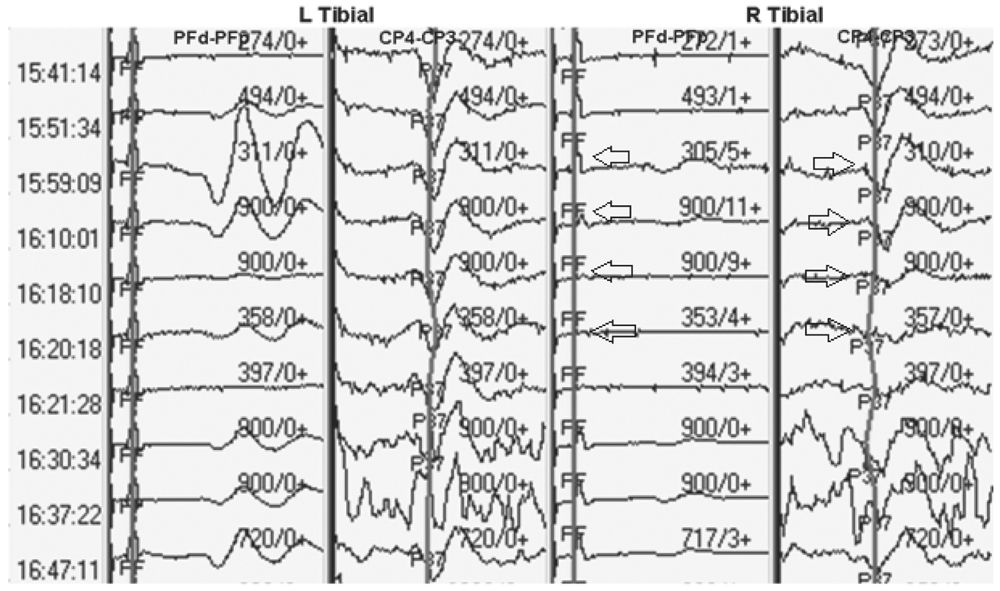
Figure 16.6: SEP recording showing loss of peripheral and cortical responses due to ischemia of the right tibial nerve (arrows).
Acute Trauma
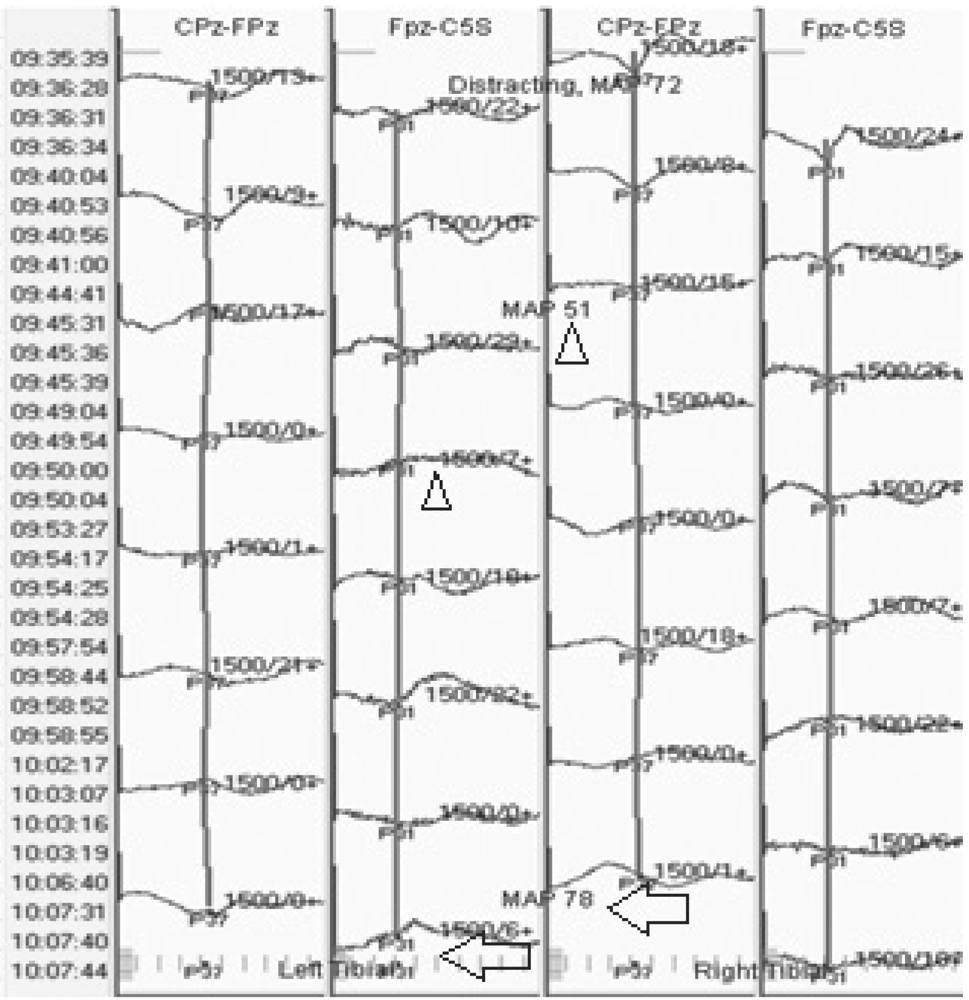
Acute trauma that is not severe enough to cause axonal disruption can cause an increase in extracellular potassium (9). The more severe the trauma, the more remarkable the increase in potassium. The extracellular potassium inhibits conduction of action potentials. As a result, with trauma, there is loss of amplitude and prolongation of latencies of SEP waveforms. This is the mechanism by which transient mechanical injury to the spinal cord can be detected with NIOM; correction of the problem can lead to complete recovery (Fig. 16.7). However, with severe or prolonged injury, axonal damage can occur, resulting in more significant changes in SEP and possibly irreversible deficits.
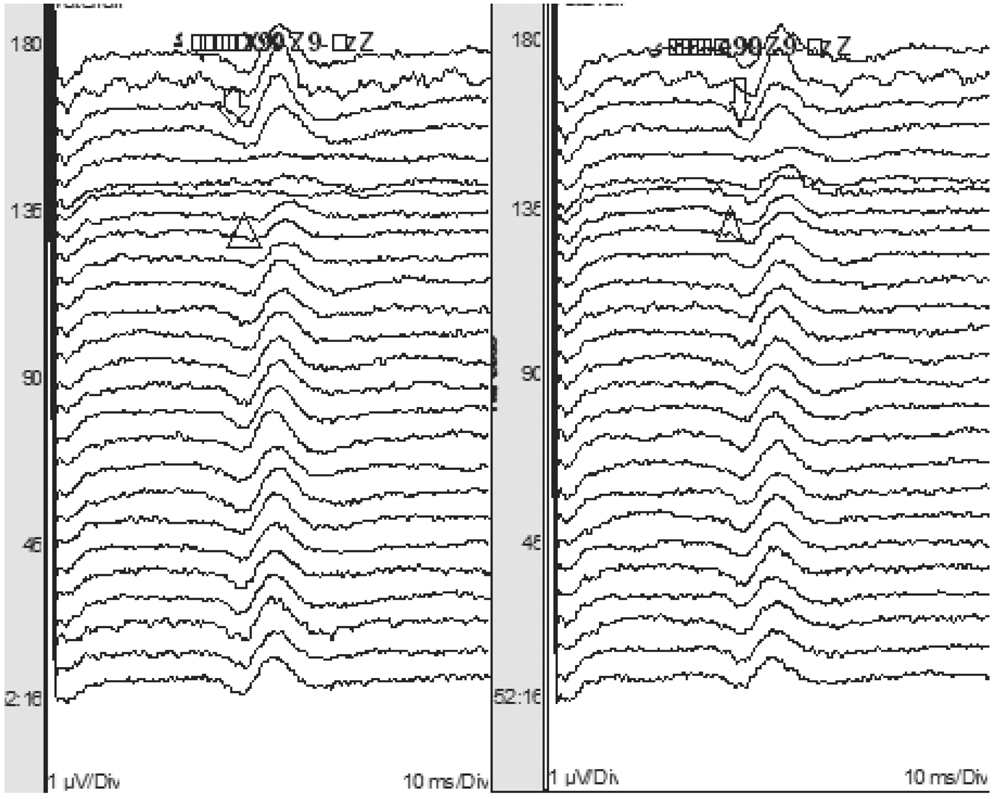
Figure 16.7: SEP recording showing loss of subcortical waveforms after stimulation of both tibial nerves with excessive distraction during scoliosis surgery (triangles). The responses improved with release of the hardware (arrows). Note that the bottom of the figure is earlier in the surgery and the top is later in the surgery.
Temperature
A drop in temperature has remarkable effects on nerve conduction and SEP. The absolute and interpeak latencies are prolonged. Amplitude is affected less with temperature changes. Soon after the patient is anesthetized, the body temperature drops, and this may shift SEP latencies and alter amplitudes if baselines were obtained immediately after induction (Fig. 16.8). In such a circumstance, it may be appropriate to reset the baseline after the temperature has stabilized. If cold irrigation is used during spinal surgery, the spinal cord may be cooled directly. This may significantly prolong latencies of the SEP waveforms. The cortical potentials are affected most by temperature, with the N20 waveform prolongation being 1.56 milliseconds for every degree Celsius drop in temperature (12).
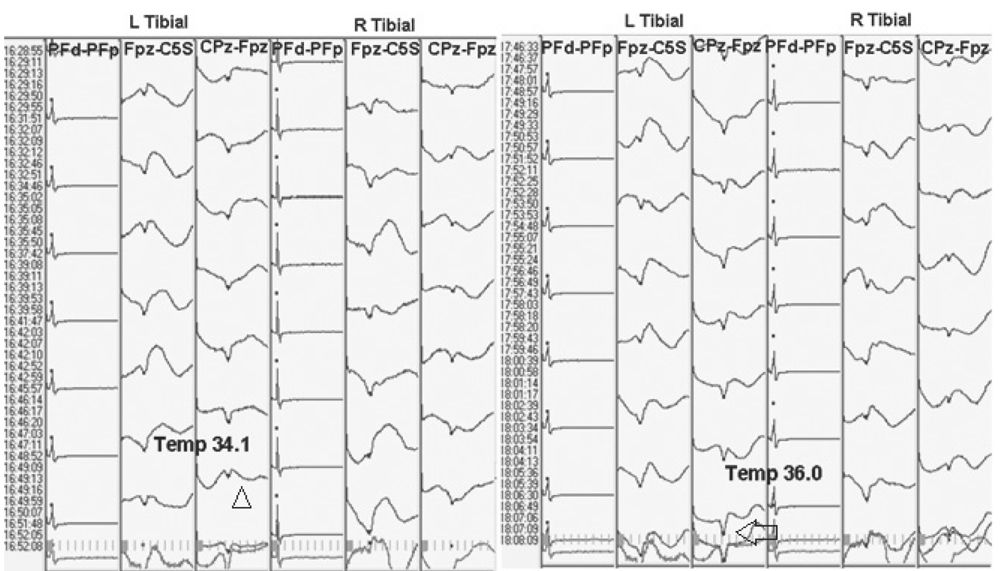
Figure 16.8: SEP recording showing a prolongation in latency and decrease in amplitude of the cortical potential with a drop in temperature (triangle). Toward the end of surgery, the temperature returns to baseline, and the latency and amplitude of the cortical response improve (arrow).
Technical Issues
Technical issues are a common cause of significant SEP change in spinal surgeries. These must be recognized and fixed if possible. The myriad of technical problems that can occur are beyond the scope of this chapter. Several broad categories should be remembered. Problems may occur with the stimulating system ranging from the software to the stimulator. The recording system, in particular the amplifiers, may be at fault. Factors local to the stimulating and recording electrodes should also be considered. Edema or ischemia around the site of stimulation may prevent adequate stimulus delivery (Fig. 16.9). The electrodes may have been dislodged. Similarly edema at the recording site, such as scalp edema, may cause a decrease in the amplitude of the response. Artifacts, such as electrocautery, can cause obliteration of the SEP response if averaging is not stopped during its use. Technical issues must be ruled out first when SEP changes are noted.
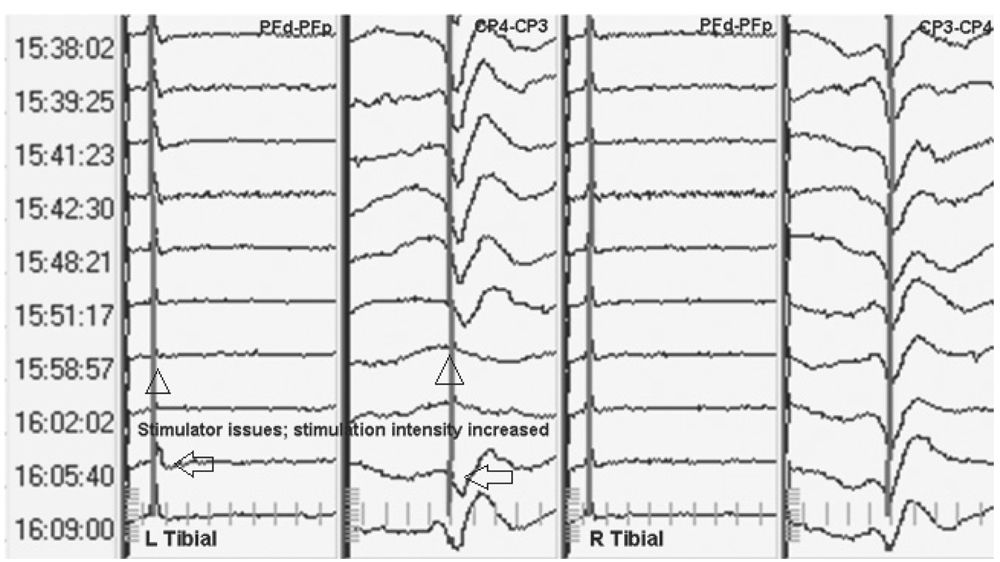
Figure 16.9: SEP recording showing loss of peripheral and cortical response after stimulation of the left tibial nerve due to local edema (triangles). Increasing stimulation intensity results in return of the SEP (arrows).
Warning Criteria
The surgeon should be alerted to changes in SEP if they are of sufficient magnitude to result in tissue injury. How much the SEP needs to vary before the change is considered significant is unclear. Somewhat, empirically, an amplitude drop greater than 50% and latency prolongation greater than 10% is considered significant (6). A change in amplitude is more significant than a change in latency. A change in SEP that is considered significant should be reproduced to ensure that the change was not due to artifact. The need to confirm a change has to be balanced with the need to immediately inform the surgeon so that corrective action can be taken immediately.
Brief or transient loss of SEP is compatible with a good outcome. Even with a complete and persistent loss of SEP, only 50% of patients will have motor weakness (13). This is considered acceptable as in NIOM specificity is often compromised for sensitivity. To minimize “false-positive” SEP changes, subcortical waveforms can be evaluated. They are more resistant to anesthetic and other physiologic changes that may occur during surgery. However, when TIVA is being used, cortical SEP can be used and are preferred. This is because anesthetic agents used in TIVA do not suppress the cortical waveform significantly. Its large amplitude allows a favorable SNR and quicker reproduction of the response.
When SEP changes are noted, the cause can be technical, physiological, or surgical. An attempt must be made to eliminate technical and physiological causes for the change before ascribing it to the surgery. As discussed previously, many technical factors can cause significant changes in SEP. Examples include machine problems, stimulator problems, reduction of stimulation intensity, and problems with recording electrodes, to name a few. Physiological causes such as anesthetic change, blood pressure fluctuations, temperature variability, and preexisting conditions can affect the response. Some inadvertent physiological changes, such as excessively low blood pressure, which compromises spinal cord perfusion, can be detected by changes in SEP. Excessive muscle activity can reduce the SNR of the subcortical potentials, reducing their amplitude. These changes can help alert the anesthesiologists to excessively low blood pressure or to a patient whose anesthesia is too light. Of course, surgical manipulation, intentional or inadvertent, of neural tissue can also cause changes in the SEP. A list of nonsurgical and surgical causes of SEP change is presented in Table 16.6.
List of Causes of Significant SEP and MEP Change
Nonsurgical causes | Surgical causes |
Anesthesia | Trauma to cord |
Blood pressure | Traction |
Temperature | Cord compression |
Preexisting conditions | Cord stretch with spinal distraction |
Variability of response | Vascular insufficiency |
Embolism or thrombosis |
Clinical Applications
There are several types of surgeries for which there are unequivocal data that SEP NIOM reduces neurologic morbidity. A recent American Academy of Neurology (AAN) Practice Parameter established the utility of SEP (and MEP) NIOM in a variety of spinal surgeries (14). A few of the types of surgeries for which SEP and MEP NIOM are useful are discussed here.
A survey of the Scoliosis Research Society showed that SEP monitoring in scoliosis significantly reduced the incidence of all neurological deficits from .72% to .55% (13). Moreover, this study showed that centers with more experience with SEP monitoring had better outcomes than those with little experience. For many other types of spine surgeries, such as anterior cervical decompression and fusion and intra and extramedullary spinal cord tumor surgery, there are smaller, uncontrolled series documenting utility of SEP NIOM.
SEP monitoring has also been used for surgeries beyond the spinal cord and vertebral column. For surgery for brainstem tumors, median nerve SEP monitoring is used in conjunction with cranial nerve monitoring. SEP can be used during cerebral aneurysm surgery to confirm that clipping does not cause critical ischemia. In surgery on descending aortic aneurysms, vascular supply to the spinal cord is often interrupted, and SEP monitoring can be used to alert the surgeon when this is occurring. Few data are available for use of SEP in nerve root, peripheral nerve, plexus, tethered cord, and movement disorder surgeries.
MEP are obtained by stimulating the motor pathways rostral to the site of surgery. Activation of the motor pathways can be measured by recording waveforms as the impulse descends in these fiber tracts. Presence of waveforms below the site of surgery confirms integrity of the motor pathways.
MEP and SEP monitoring are often performed together as they provide complementary information. Whereas SEP assess the dorsal column pathways and the posterior aspect of the spinal cord, MEP monitoring provides information about the function of the corticospinal pathways and the lateral and anterior spinal cord. Before MEP monitoring was routinely performed, SEP monitoring alone was used during surgeries in which the spinal cord was at risk. It was assumed that SEP served as a surrogate marker of injury to motor pathways. Several studies, however, showed that motor deficits could occur without any change in SEP signals (13,15). Since MEP directly assess motor pathways, they provide a more sensitive marker of injury to these pathways.
Anatomy and Physiology
Stimulating the motor pathways in the brain or spinal cord can evoke MEP. For many years, MEP monitoring was performed by electrically stimulating the spinal cord rostral to the site of surgery and recording muscle MEP from the lower extremities. Stimulation was performed with an electrode placed in the epidural space during surgery or percutaneously before the start of the procedure. It was thought that epidural electrical stimulation of the spinal cord resulted in activation of the motor pathways. However, it has been shown with collision studies that this type of stimulation results in retrograde activation of the dorsal column axons. Collaterals from these axons synapse on anterior horn cells and activate muscle action potentials. Consequently, motor pathways in the spinal cord remain unmonitored with this technique.
Transcranial stimulation results in the activation of motor neurons in the cortex. This provides selective activation of the motor pathways and motor pathway monitoring. Transcranial stimulation of motor pathways can be accomplished with magnetic or electrical stimulation. Magnetic stimulation involves high-intensity magnetic stimulation delivered with a coil placed near the cortex. The magnetic stimulus results in depolarization of cortical interneurons, which results in activation of the motor pathways. It is painless and can be performed in an awake patient. However, transcranial magnetic stimulation is very sensitive to anesthetics because of its reliance on interneurons, and involves the use of bulky stimulating equipment. These issues prevent its routine use during surgery.
In the last few years, transcranial electrical stimulation (TES) has become available as a means for obtaining MEP. A single electrical stimulus delivered to the cortex, directly or through the scalp, results in the activation of the cortical pyramidal neurons and a descending corticospinal tract volley. This volley travels in the corticospinal tracts in the midbrain and pons and then decussates to the contralateral side in the medulla. In the spinal cord, the corticospinal tract has lateral and anterior components. The lateral corticospinal tract contains most of the descending motor fibers. Fibers from both lateral and anterior corticospinal tracts synapse on the anterior horn cells of the ventral roots of the spinal cord gray matter. From here arise motor fibers that travel through anterior roots of the spinal nerves to form motor nerves that innervate muscles.
The descending volley of depolarization produced by activation of the cortical pyramidal neurons can be recorded as a “D” (direct) wave with an epidural electrode placed on the spinal cord. Following the D wave, several “I” (indirect) waves can also be recorded, and they represent activation of cortical interneurons, which cause recurrent excitation of the corticospinal pathway.
A single transcranial electrical stimulus does not result in sufficient depolarization of alpha motor neurons to cause a muscle (“M”) MEP that can be recorded from the extremities. However, a train of three to nine electrical stimuli applied at 2- to 8-millisecond intervals results in summation of depolarization on the alpha motor neurons, resulting in their activation. This results in a recordable muscle MEP response from the extremities. It is this train of stimuli and recording of muscle MEP responses that is used most often when contemporary MEP monitoring is performed.
Though most types of stimulation in clinical neurophysiology are cathodal, with TES anodal stimulation is more effective. The cell body and axon hillock, the sites of stimulation for TES, are more sensitive to anodal stimulation. Thus, anodal stimulation is used during TES and is more reliable than cathodal TES.
Both the lateral and the anterior corticospinal pathways are in the anterior two-thirds of the spinal cord and are supplied by branches of the anterior spinal artery (see earlier discussion). Consequently, selective injury to this vascular supply results in injury to the anterior two-thirds of the cord, resulting in motor weakness with preservation of sensory modalities mediated by the dorsal column pathways.
Methodology
The methods used to stimulate motor pathways and techniques used to record the descending volley of activation will be discussed in this section. The techniques used most commonly will be discussed in detail.
Stimulating Techniques
MEP can be obtained by stimulating the motor pathways at the level of the cortex or spinal cord. Because of the issues discussed earlier with spinal cord stimulation, this technique is not used regularly and will not be discussed further. Magnetic transcortical stimulation will also not be discussed further due to its limited utility during surgery. The remainder of the discussion will focus on TES.
Stimulating Equipment
A stimulator that is used for TES must be capable of delivering a high-intensity electrical stimulus to the scalp. Constant-voltage stimulators are used most often, and most have the capacity to deliver at least 1,000 V and a train of nine rectangular pulses. The actual current delivered depends on the impedance of the electrodes being used for stimulation. Digitimer was the first stimulator to obtain FDA approval for TES and was designed specifically to deliver a high-intensity TES. Other manufacturers have subsequently made similar stimulators. Currently, many brands of commercially available NIOM equipment offer built-in TES capability (Table 16.7).
Characteristics of Commercially Available TES Equipment

Stimulating Electrodes
Several types of stimulating electrodes can be used to deliver the electrical stimulus to the scalp. Spiral needles (also known as corkscrew electrodes), straight needles, and surface EEG cup electrodes have been used. The average impedances of these electrodes are 500, 800, and 1,100 Ω, respectively. The impedance of the electrodes is an important consideration as the MEP threshold is directly proportional to the electrode impedance above 460 Ω (16). Consequently, higher current will be needed to elicit MEP when surface EEG cup electrodes are used compared with spiral needles. Most commonly, spiral and straight needles are used. Pictures of these electrodes are presented in Figs. 16.1 and 16.2, and their advantages and disadvantages are presented in Tables 16.2 and 16.3.
Electrode Placement
Stimulating electrodes are placed on the scalp at sites that overlie the motor cortex as specified by the International 10–20 system. Typically, electrodes are placed at C1 and C2 or C3 and C4 locations (Fig. 16.10) (17,18). At times, displacing electrodes 1 to 2 cm anterior to these sites results in better responses. Additionally, electrodes can be placed at Cz − 1 cm and Cz + 6 cm. Montages that can be used for stimulation and their advantages are listed in Table 16.8.
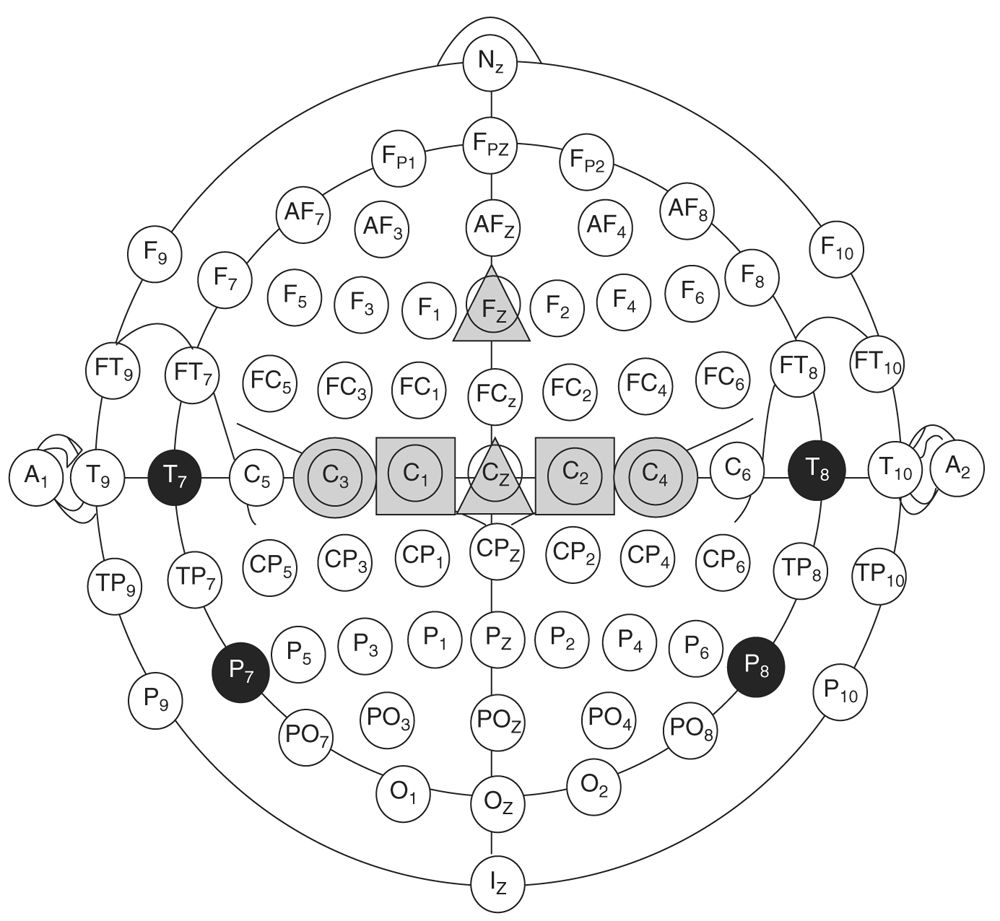
Figure 16.10: Possible locations for placing MEP stimulating electrodes. Common sites are at C1 and C2 (gray squares) or C3 and C4 locations (gray circles). Moving the electrodes 1 to 2 cm anterior to these landmarks may result in better responses. Electrodes at Cz − 1 cm and Cz + 6 cm (gray triangles) have also been shown to result in good responses in some patients. (Adapted from reference Journee HL, Polak HE, de Kleuver M. Influence of electrode impedance on threshold voltage for transcranial electrical stimulation in motor evoked potential monitoring. Med Biol Eng Comput 2004;42(4):557–561. Epub August 24, 2004.)
MEP Stimulating Montages and Their Advantages
Montage | Advantage |
C1-C2/C2-C1 | Best for obtaining lower extremity responses |
C3-C4/C4-C3 | Best for obtaining upper extremity responses |
C3-Cz/C4-Cz | Best for obtaining lateralized responses |
Cz − 1 cm − Cz + 6 cm | Best for obtaining bilateral lower extremity responses |
Most commonly, MEP monitoring is performed with C1-C2/C2-C1 or C3-C4/C4-C3 pairs. Using the latter pair may result in deeper, axonal stimulation of the motor pathways and shorter D wave latencies. Often, the high current intensity used for MEP results in a large stimulus artifact, making it harder to identify a response. Disconnecting the SEP recording electrodes from the head box or moving the MEP stimulating electrodes 1 to 2 cm anterior to their typical location may reduce the stimulus artifact. Some stimulators have switch boxes that automatically disconnect SEP electrodes when the electrical stimulator is activated and vice versa to minimize artifacts.
Stimulation Parameters
As noted previously, a train of stimuli is needed to evoke a muscle MEP. A train of at least three stimuli is needed, and most stimulators can deliver up to nine stimuli. In a patient with a normal nervous system, three to four stimuli are enough to elicit a muscle MEP in the hand, and five stimuli are needed for leg MEP. The exact number of stimuli needs to be adjusted during the surgery to maximize the responses of interest.
The interval between the stimuli, the interstimulus interval (ISI), can be adjusted between 1 and 8 milliseconds. Longer ISI allow summation of all the I waves. However, the depolarization produced by the D waves begins to subside by the time all I waves summate. When the ISI is too short, subsequent stimuli may fall within the absolute or relative refractory period of the pyramidal neurons, causing a diminished response. Thus, like the number of stimuli, the ISI must be individualized to maximize the response. With light anesthesia that allows expression of I waves, long ISI may be preferable. With standard anesthesia, an ISI of 4 to 5 milliseconds may be optimal. A short ISI of 2 milliseconds has been shown to result in optimal hand muscle MEP (19). In most cases, when both upper and lower extremity MEP monitoring is needed, an ISI of 4 milliseconds, a train of five stimuli, and a stimulation intensity of 300 V serve as a good starting point (18).
Facilitation
Occasionally, a muscle MEP response may be difficult to obtain in the lower extremity. Facilitation refers to enhancing the depolarization at the anterior horn cells prior to the target stimulus arriving. This can be done by delivering a priming stimulus to the anterior horn cells. Manual stimulation of the sole of the foot can provide such a priming stimulus, but this is difficult to perform in the operating room. There are more practical methods of delivering the priming stimulus during surgery.
Double Train Stimulation. As the name implies, two trains of stimuli are applied to the scalp in rapid succession. The first, priming train precedes the target train by a set interval. The priming train causes partial depolarization of the anterior horn cells, and the target train completes the depolarization, resulting in a muscle MEP. Both trains can have similar stimulation parameters, or the priming train may have fewer stimuli. The intertrain interval (ITI) should be less than 10 milliseconds or greater than 100 milliseconds. ITI between 10 and 100 milliseconds has been shown not to result in significant facilitation (20).
Posttetanic Stimulation. Tibial nerve stimulation can also depolarize the anterior horn cells by antegrade transmission of the stimulus through motor nerve fibers. This depolarization can result in facilitation of the MEP as well. Tetanic stimulation of the tibial nerve at 50 Hz for 5 seconds followed 1 second later by MEP stimulus has been shown to result in higher-amplitude MEP responses in not only tibial innervated muscles, but muscles in the other limbs as well (21). Many new NIOM machines can be programed to deliver the tetanic stimulus followed by a MEP stimulus.
Recording Techniques
MEP can be recorded from the spinal cord, nerves, and muscles. Each recording site requires specific electrodes and has advantages and disadvantages.
Recording Electrodes
Recording electrodes are selected depending on the site of recording. Specialized epidural electrodes are available, which can be inserted percutaneously or placed intraoperatively by the surgeon. These electrodes will record D and I waves. Surface and needle electrodes are used to record MEP from nerves and muscles. Surface electrodes can be applied in an awake patient, but application is time consuming. Needle electrodes are applied after the patient has been anesthetized and can be inserted directly into the muscle of interest. When recording MEP from nerves, needle electrodes can be inserted in close proximity to the nerve, resulting in a higher-amplitude response. The advantages and disadvantages of various types of electrodes are presented in Tables 16.2 and 16.3. Nerve MEP are seldom recorded and will not be discussed further.
Spinal Cord Recordings
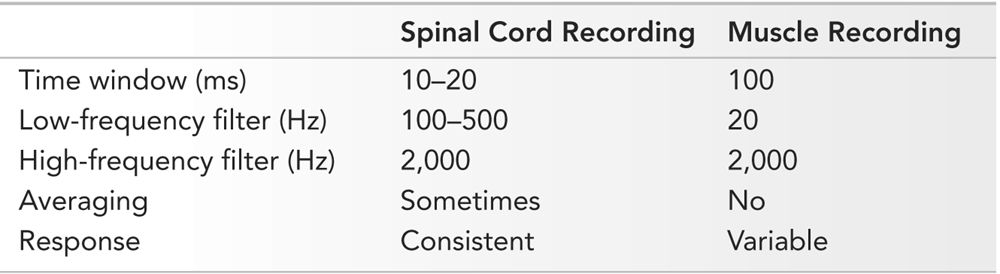
Typical parameters for spinal cord recordings are listed in Table 16.9. As the stimulation intensity increases, the D wave amplitude increases and latency decreases. I waves start to appear with still higher stimulation intensity, and finally the D wave becomes bi-fed. For optimal recording, the stimulation intensity should be increased to maximize the amplitude of the D wave but not so high that it becomes bi-fed or polyphasic. D waves are most easily recorded in the cervical region and are difficult to record at lumbosacral levels. Reliable D waves have not been recorded in children less than 21 months of age (18). At times, D waves are of low amplitude and have to be averaged to discern a reproducible response. In such situations, 10 to 20 repetitions are usually enough to obtain a response.
Typical Spinal Cord and Muscle MEP Recording Parameters
The advantages of D wave recordings are their high SNR, reproducibility, and constant morphology. Often a single stimulus, rather than a train, can elicit a D wave response; though, as noted earlier, sometimes D waves need to be averaged. Additionally, anesthetics have little effect on D waves. However, the recording electrodes must be placed by the surgeon or percutaneously in the epidural space. The electrode, because of its size, cannot be placed in the high cervical region, and recordings are not possible in the lumbosacral region, thereby limiting the sites from where D waves can be recorded. Demyelination or tumors within the spinal cord may cause temporal dispersion and desynchronization of D waves, making them not recordable.
Muscle Recordings
Muscle MEP recordings are used most commonly. Typical parameters are listed in Table 16.9. They are most often recorded with needle electrodes, which result in polyphasic responses. Subsequent responses are variable and dependent on how many and which motor units were activated. Consequently, averaging is not performed. Muscle MEP are most easily recorded from distal limb muscles, such as the abductor pollicis brevis, abductor digiti minimi, abductor hallucis, and tibialis anterior. A typical montage for recording muscle MEP is presented in Table 16.10.
Typical Muscle MEP Recording Montage
Upper Limb | Lower Limb |
APB-ADM | AH-ADQ |
FCU-FCR | AH-TA |
TA-TA | |
TA-MG | |
VM-VL |
APB, abductor pollicis brevis; ADM, abductor digiti minimi; FCU, flexor carpi ulnaris; FCR, flexor carpi radialis; AH, abductor hallucis; ADQ, abductor digiti quinti; TA, tibialis anterior; MG, medial gastrocnemius; VM, vastus medialis; VL, vastus lateralis.
There are several advantages of recording muscle MEP over spinal cord MEP. Muscle MEP can be recorded with needle electrodes that can be placed by the monitoring staff. Several muscle groups can be monitored, and selective localization of injury is possible. When D waves are not recordable, as with tumors, muscle MEP may still be present. The disadvantages of muscle MEP include their variable morphology, which precludes averaging, high sensitivity to inhalational anesthetics and NMJBA, and lack of consistent interpretation criteria. An example of muscle MEP recordings is presented in Fig. 16.11.

Figure 16.11: Example of a muscle MEP. Notice the variable morphology of successive responses. This variable morphology precludes averaging of muscle MEP.
Anesthetic Considerations
Anesthesia has a profound effect on MEP monitoring. As noted earlier, magnetic MEP monitoring cannot be performed in the operating room since the anesthetic agents prevent adequate stimulation. Anesthetics affect synapses and consequently have a greater effect on MEP, which are dependent on synaptic transmission.
Inhalational Agents
All inhalational agents suppress cortical neurons and anterior horn cells. This makes obtaining muscle MEP difficult and unreliable (Fig. 16.12). Approximately 60% of neurologically normal patients have recordable muscle MEP at 0.6% isoflurane, and only 20% have MEP at 0.8% isoflurane (22). A low concentration of an inhalational anesthetic, usually less than 0.5%, may be permissible with MEP monitoring. However, if responses are small and unreliable, alternate anesthetics should be considered. Inhalational anesthetics have no appreciable effect on D wave recordings.
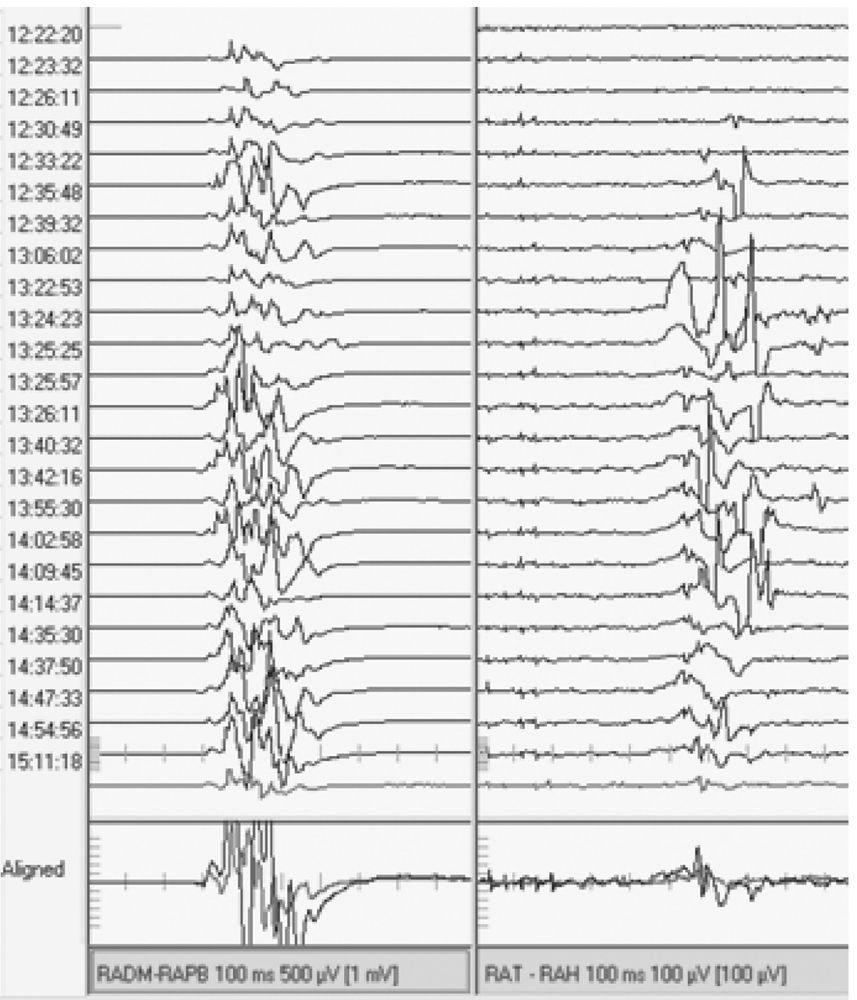
Figure 16.12: MEP responses from right upper (left) and lower (right) extremities. Isoflurane (1%) was being used toward the start of this tracing (top) and was discontinued around 12:26:11. Notice the appearance of reproducible muscle MEP responses in the lower extremity after isoflurane was discontinued.
Intravenous Agents
Propofol is the IV anesthetic used most often with MEP monitoring. Doses up to 25 mg/kg/hour do not cause significant changes in the MEP amplitude. When compared with inhalational agents, doses of propofol causing comparable suppression of the bispectral index (BIS) do not cause any change in MEP, while inhalational agents make them mostly not recordable (22,23). Propofol is supplemented by a narcotic analgesic such as fentanyl. In doses typically used in the operating room, fentanyl and similar narcotic analgesics do not cause significant changes in the MEP. When robust muscle MEP must be recorded, a TIVA technique is useful, incorporating propofol and a narcotic analgesic.
Neuromuscular Junction Blocking Agents
When muscle MEP are recorded, NMJBA should be used only with caution. Persistent use of these drugs will eliminate the muscle MEP. However, D waves can still be recorded. If TES produces excessive movement that must be limited, a partial neuromuscular blockade can be used with an IV infusion of a short-acting NMJBA. Intermittent boluses of a NMJBA should not be used as that causes excessive variability of the MEP, making monitoring unreliable. When an infusion is used, at least two out of four twitches must be present in a train-of-four (TOF) stimulation for reliable MEP monitoring (24). The TOF should be monitored by the NIOM team in the limb from which the muscle MEP is being recorded. The use of partial neuromuscular blockade, however, adds considerable complexity to monitoring and risks, making MEP monitoring unreliable if the infusion of the NMJBA is not tightly controlled. Administration of magnesium and α2-receptor agonists such as ketanserin can also potentiate neuromuscular blockade and suppress muscle MEP amplitudes (25).
Mechanisms of MEP Change
As with other types of monitoring, the main causes of change in MEP monitoring can be surgical intervention, changes in the physiologic milieu, or technical issues with the monitoring. For surgeries on the spinal cord, recording muscle MEP in the upper and lower limbs can aid in determining the etiology of the change. The upper limb MEP serve as controls in this situation; whatever affects both upper and lower limb MEP is likely a systemic factor, while when only lower extremity MEP are affected, the cause is more likely to be surgical. The most common reasons for MEP change will be discussed here. Many of the factors that cause a change in SEP can also affect MEP (see Table 16.6).
Ischemia/Blood Pressure
Ischemia of the spinal cord can result in loss of MEP (Fig. 16.13). The gray matter of the spinal cord is more susceptible to reduced blood supply than the white matter. Surgeries involving clamping of the aorta suggest that muscle MEP are lost within 2 to 5 minutes of onset of ischemia, whereas SEP take 7 to 30 minutes to be affected (3). If only the anterior spinal cord is affected by ischemia, SEP may not change at all. A drop in the mean arterial pressure during surgery can have a similar effect on MEP.
Figure 16.13: Sudden loss of lower extremity muscle MEP with preservation of upper extremity MEP during resection of tumor around descending aorta (arrow). A major blood vessel supplying the anterior spinal artery was likely inadvertently cut.
Peripheral nerves may also be affected by ischemia and result in loss of muscle MEP responses from muscles supplied by the ischemic nerve. This may occur when a tourniquet or wrapping is applied tightly around a limb, compromising the vasa nervosum. A similar situation may arise when large-bore catheters are placed in blood vessels. This may result in ischemia of the distal limb, leading to loss of MEP from that limb (26). In this situation, the muscle MEP disappears after the SEP peripheral response has disappeared.
Acute Trauma
Trauma to the spinal cord, as may occur during vertebral column manipulation, can cause MEP changes. The mechanism underlying this change has been discussed earlier with SEP monitoring. If the trauma is detected early, it may be reversible if the intervention that produced the change can be reversed.
Temperature
MEP are affected by changes in core body temperature. MEP obtained in animals with spinal cord stimulation were found to be affected if the temperature changed as little as 2°C to 2.5°C. At a temperature of 28°C, MEP cannot be recorded (27). If substantial temperature alteration occurs after the start of the surgical procedure, new MEP baselines may need to be set prior to start of the critical phase of the surgery.
Anesthetics
As discussed previously, muscle MEP monitoring is very sensitive to anesthetics. Inhalational anesthetics can significantly reduce MEP amplitude even at a low concentration. Though propofol is the preferred agent for MEP monitoring, very high doses of this can also reduce MEP amplitude. NMJBA should be used only as a continuous infusion, as a bolus will cause a sudden drop in MEP amplitude, making interpretation difficult.
Technical Issues
Many technical issues can lead to MEP changes. Common technical issues involve problems with stimulating electrodes. If straight needles are used, they can become dislodged during surgery. Not only will this result in loss of MEP, but if the needles are reinserted in a slightly different position, the MEP may appear to be of different amplitude and morphology. If the needles are closer to the C3-C4 position, the upper limb MEP amplitude may be greater, whereas if they are inserted closer to C1-C2, lower limb responses may be larger. Problems may occur with the stimulator itself. If a stimulator is used that cannot deliver sufficient charge to the scalp, MEP may not be recordable. The recording system may also cause MEP changes. If the recordings are performed with needle electrodes, these may become dislodged, changing the morphology of the response or obliterating it completely. If the SEP recording electrodes are not removed from the jack box prior to TES, a stimulus artifact may make it impossible to visualize the muscle MEP. As with other types of monitoring, technical issues must be considered before raising an alert about the change in MEP monitoring.
Warning Criteria
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree