The use of EEG in the evaluation of seizure disorders typically begins in the outpatient setting as a procedure performed using scalp electrodes during the interictal period. Ictal EEG is now commonly performed with simultaneous video recording, in either outpatient or inpatient setting. The objective of this chapter is to discuss the types of interictal epileptiform discharges (IEDs) and ictal discharges recorded from the scalp in adults. The emphasis of this chapter is on the features of IEDs and ictal discharges and their clinical correlation.
INTERICTAL EPILEPTIFORM DISCHARGES
According to the International Federation of Societies for Electroencephalography and Clinical Neurophysiology, “epileptiform” abnormalities “applies to distinctive waveforms or complexes resembling those recorded in a proportion of human subjects suffering from epileptic disorders and in animals rendered epileptic experimentally” (1). Another definition of epileptiform waveforms or patterns is that they are EEG abnormalities that are associated with a predisposition to experiencing or developing epileptic seizures. The word predisposition is used to indicate that the association between epileptiform abnormalities and seizure disorders is not absolute. Presence of epileptiform discharges does not necessarily indicate that the patient has a seizure disorder (2). However, the detection of epileptiform abnormalities does increase the likelihood of an epileptic seizure disorder being present. When the finding is taken together with the clinical history and other diagnostic test results, epileptiform abnormalities help in establishing the diagnosis of epileptic seizure disorders.
Spikes/Sharp Waves
Spikes are transient waveforms with pointed peaks when displayed at a screen resolution of approximately 30 mm per second (Fig. 11.1). By definition, duration of spikes varies from 20 to 70 milliseconds, whereas sharp waves are wider with duration between 70 and 200 milliseconds (1). The distinction in this regard between spikes and sharp waves is somewhat arbitrary. The two types of waves often occur in the same clinical disorder or the same patient.
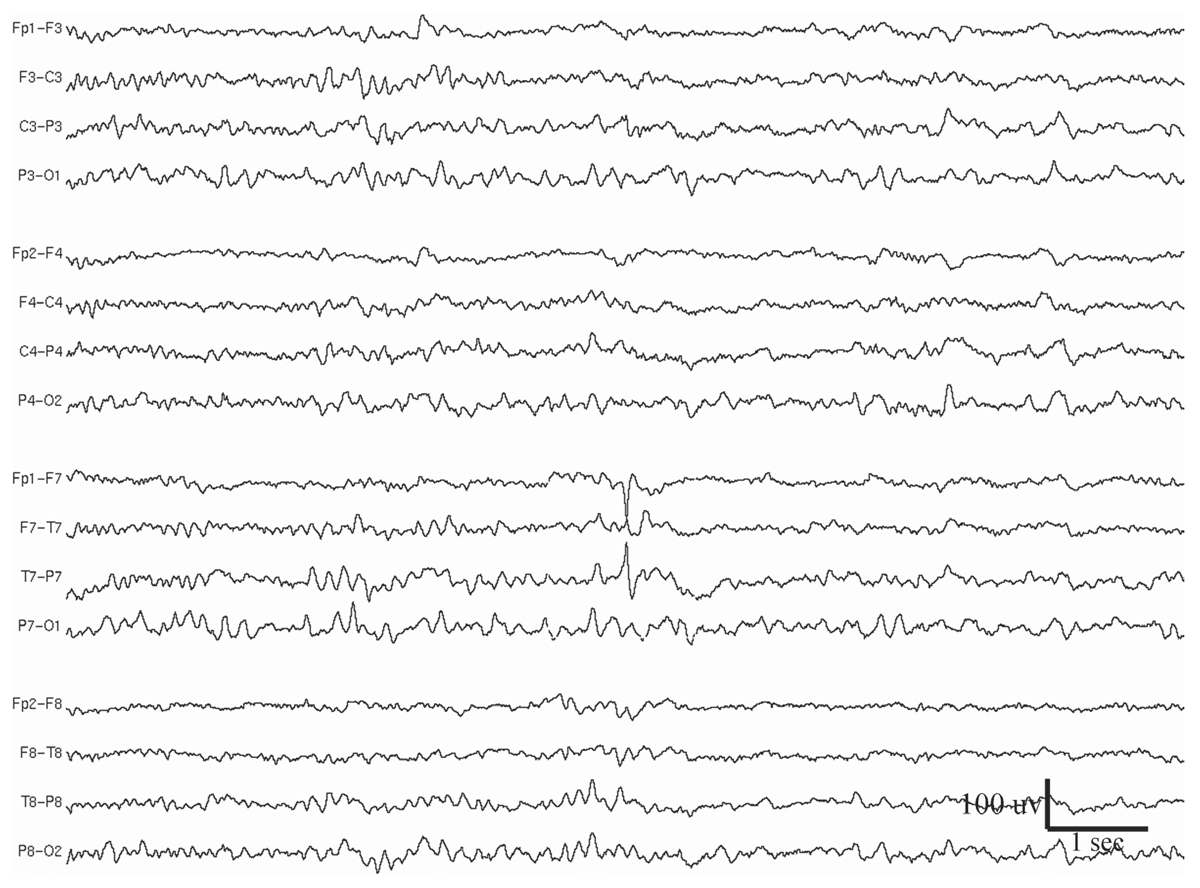
Figure 11.1: Left midtemporal spike in a 33-year-old woman who had been experiencing spells of smelling burnt rubber, followed by lip smacking and behavior of confusion. This spike discharge is not followed by an after-going slow wave. MRI showed a cavernous angioma at the left temporal lobe, despite lack of left temporal slowing during wake EEG.
Spikes/sharp waves should have sufficient amplitudes to distinguish them from the background, such as by a factor of two. They are often polyphasic, but the main component is usually surface-negative. Surface-positive IEDs are rare, and can occur at the site of craniotomy (3), particularly if lateral convexity cortex is removed, but not more mesial cortex. Central positive sharp waves can be observed in infants with intraventricular hemorrhage or periventricular white matter injury, but the discharges are a better indication of encephalopathy than of epileptogenicity (4).
Spikes/sharp waves are often followed by a slow wave that can be smaller or larger than the spike/sharp wave discharges themselves. The spike/sharp wave with its after-going slow wave may be referred to as a “spike-and-slow-wave complex” (i.e., a complex is a series of two or more individual waves). The accompaniment of spikes/sharp waves by an after-going slow wave may not be constant in the same patient and at the same location. When the spike/sharp wave is of negative polarity, as is most often the case, the after-coming wave will be negative as well. Separating these two will be a deflection of positive polarity.
Focal Spikes/Sharp Waves
Common locations of focal spike/sharp-wave discharges, in the approximate order of frequency, are temporal, frontal, centrotemporal, parietal, occipital, and midline central and/or paracentral. The clinical correlation of focal spikes/sharp waves is with focal epilepsy; however, the likelihood that a given focal spike/sharp wave is associated with epilepsy varies with its location (5). For instance, the association is higher for temporal spikes/sharp waves than for rolandic or occipital spikes/sharp waves. Approximately 90% of children with anterior temporal spikes have seizures, whereas seizures are present in only 38% of those with rolandic spikes. Temporal lobe tissues, especially the hippocampus and the amygdala, are some of the most epileptogenic. The temporal lobes are also frequently involved in pathologic conditions, such as hypoxia, strokes, tumors, trauma, and vascular malformations. In contrast, many children with occipital IED do not have epilepsy; in fact, about 60% of children with occipital spikes do not have epileptic seizure disorders (6), and occipital IEDs are even encountered in nonepileptic persons with migraine disorders (7). Other occipital discharges, known as “needle spikes,” can be observed in the EEG of children who have congenital blindness but not epilepsy (8). These occipital spikes are low in amplitude and sharp in configuration.
Most focal spikes/sharp waves are surface negative at the scalp. Positive spikes/sharp waves are not common in the adult patient. They can be seen postoperatively when overlying convexity cortex is removed, but more mesial cortex remains. Synchronous, but spatially separated, positive and negative spikes are evident when the spike voltage field is tangential rather than radial. Positive-polarity discharges are more frequently encountered in newborns with periventricular hemorrhage or leukomalacia and in young children with multifocal spikes/sharp waves, especially in the presence of global encephalopathy, such as with ischemic injury or lipid storage diseases (9,10).
The identification of focal spikes/sharp waves is very important in the diagnosis of benign, age-related epilepsy syndromes (see Chapter 10). Spikes/sharp waves in these syndromes have distributions, morphology, and activation factors that are characteristic for each syndrome. The most common is benign epilepsy of childhood with centrotemporal spikes (also known as benign epilepsy with centrotemporal spikes, BECT, or benign rolandic epilepsy). Other childhood syndromes of focal epilepsy are benign childhood epilepsy with occipital paroxysms and the syndrome of early-onset childhood seizures with occipital spikes (Panayiotopoulos syndrome) (11).
Multifocal Spikes
Multifocal spikes/sharp waves refer to the presence of multiple independent foci of spikes or sharp waves that involve both hemispheres (Fig. 11.2). Although this abnormality can be seen at any age, it is most frequently observed in children aged 4 to 7 years (12). Background EEG slowing is present in nearly all these patients, and 94% of them have seizures. Generalized motor seizures are the most common, occurring in 76% of patients (13). Seizures are typically frequent, with half of the patients experiencing daily seizures. Concomitant neurological abnormalities are also common; 45% of these patients have motor deficits and 82% have mental retardation and developmental delay. The majority (71%) have underlying structural brain abnormalities or a history of brain injury.
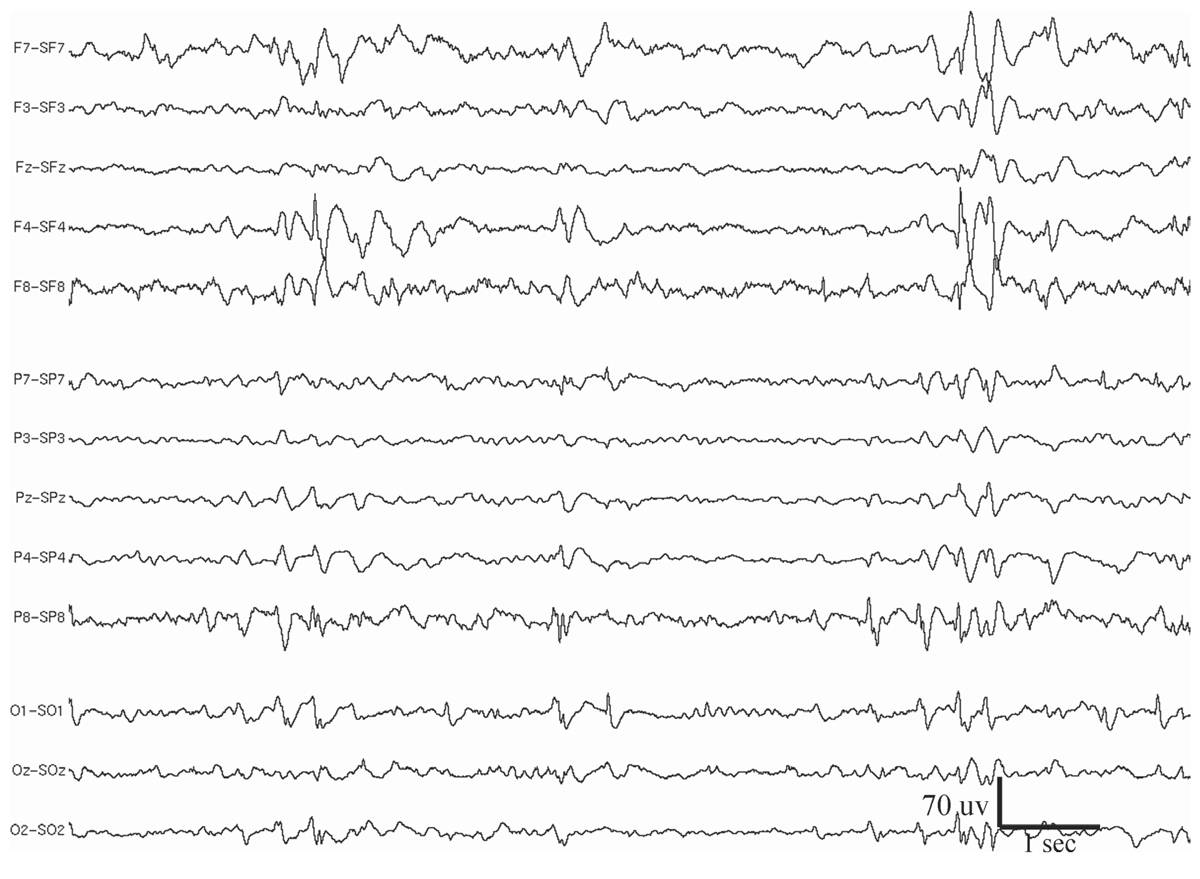
Figure 11.2: Multifocal spikes appearing at F4, F8, P8, O1, and bisynchronously at the occipital regions. The patient had medically refractory seizures since childhood. Note also the generalized slowing in the background. EEG is displayed in Laplacian montage. The patient had other EEG abnormalities, which, together with the clinical history, suggest LGS (see Figs. 11.7 and 11.9).
Periodic Lateralized Epileptiform Discharges
Periodic lateralized epileptiform discharges (PLEDs) are epileptiform discharges or complexes that recur with a regular periodicity in one hemisphere, usually every 0.3 to 4 seconds (14) (Fig. 11.3). This periodic activity may take the form of monophasic or polyphasic spikes or sharp waves, which may or may not have accompanying slow waves (15,16). Although very focal PLEDs have been observed, usually the discharges are regional in distribution within a hemisphere, or more diffusely affecting a whole hemisphere. The temporo-parietal-occipital region is most frequently involved. PLEDs are highly associated with acute cerebral disorders, especially those producing structural lesions such as stroke (infarct or hematoma), brain trauma, herpes encephalitis, tumor, and abscess. Rare causes include metabolic encephalopathy, Creutzfeldt-Jakob disease, migraine, and toxic encephalopathy (e.g., aminophylline or alcohol intoxication).
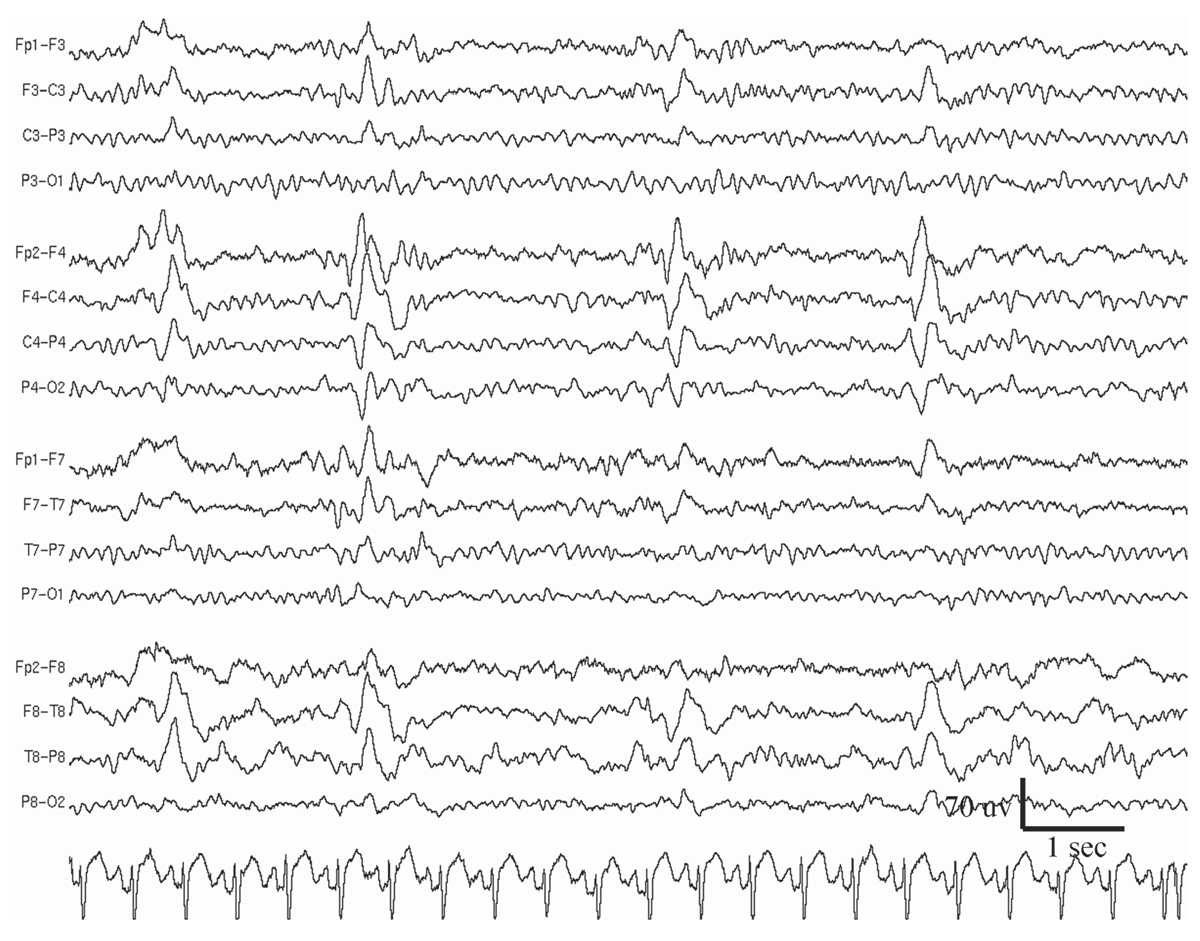
Figure 11.3: Periodic lateralized epileptiform discharges (PLEDs) at the right hemisphere in a 40-year-old man who had a small right frontal tumor. The patient experienced a generalized convulsive seizure 12 hours before the EEG recording was performed.
Fifty percent of patients with PLEDs will develop seizures, most often of a focal nature, with or without secondary generalization. Reiher and colleagues (17) observed that PLEDs may be multiphasic and burst-like in appearance. PLEDs-plus is the term they coined for this type of periodic activity, as opposed to the less complex morphology of PLEDs-proper. PLEDs-plus carries a much higher association with clinical seizures and status epilepticus. In fact, ictal activity is commonly recorded with PLEDs-plus.
PLEDs are transient and they may transform over days or weeks into intermittent monomorphic slow waves with or without sporadic spikes/sharp waves. The interval between discharges typically lengthens over time as well. These intermittent slow waves eventually disappear, possibly leaving residual focal slowing, which reflects the sequela of underlying brain damage.
When PLEDs are recorded bilaterally, the term BIPLEDs is commonly used. These discharges may be temporally dependent or independent (18). They are encountered in patients with severe hypoxic encephalopathy or bilateral hemisphere destructive lesions. BIPLEDs, particularly when independent, are associated with a poor prognosis for survival or recovery of normal neurological functions.
In multifocal PLEDs, there are at least three foci of periodic activity involving both hemispheres (19). Multifocal PLEDs are encountered in patients with severe and diffuse brain dysfunction or with multifocal lesions of both cerebral hemispheres. Etiologies include multifocal strokes, infection, a state of seizure exacerbation, and toxic/metabolic encephalopathy. Nearly 90% of patients with multifocal PLEDs have seizures. Prognosis is dependent on the underlying cerebral disorder. For example, patients with acute cerebral lesions or infections have a higher mortality than those whose PLEDs follow a bout of multiple seizures.
Temporal Intermittent Rhythmic Delta Activity
Temporal intermittent rhythmic delta activity (TIRDA) consists of intermittent sinusoidal trains of rhythmic delta waves from the temporal region that typically last several seconds (Fig. 11.4) (20,21). The most common frequency is 2 to 3 Hz. Although anterior temporal is the predominant location, posterior TIRDA can also be observed. TIRDA may appear in wakefulness or sleep, but it is often most easily identified in drowsiness. Reiher and colleagues (20) have demonstrated that TIRDA is highly correlated with a history of temporal lobe seizures. In a case-control study, all patients with TIRDA were shown to have complex partial epilepsy (21), and the majority also had temporal spikes/sharp waves on their EEGs. Temporal depth electrode recording during TIRDA on the scalp showed active spiking from the amygdalohippocampal structures. TIRDA is commonly associated with underlying structural lesions. In fact, two-thirds of the patients in the study had pathology of the temporal lobe.
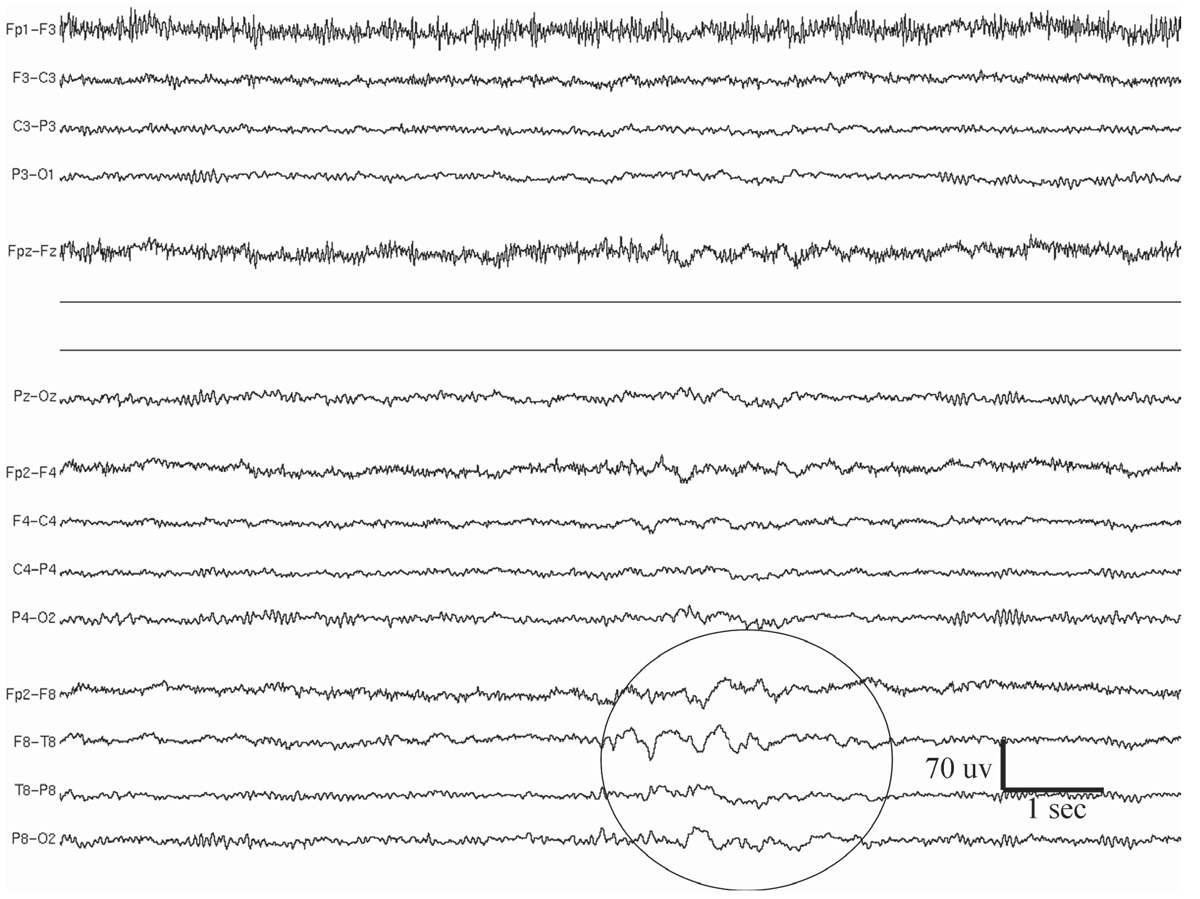
Figure 11.4: Temporal intermittent rhythmic delta activity (TIRDA; indicated by a circle) at the right temporal lobe in a 32-year-old patient with medically refractory epilepsy. MRI showed evidence of right mesial temporal sclerosis, in the form of hippocampal atrophy and fluid-attenuated inversion recovery (FLAIR) signal abnormality.
Generalized IED
Generalized IEDs have a variety of forms—3-Hz spike-and-waves, atypical spike-and-slow-waves, slow spike-and-wave discharges, hypsarhythmia, and generalized repetitive fast discharge (GRFD).
3-Hz Spike-and-Wave
As the name implies, 3-Hz spike-and-wave discharges are runs of bilateral spikes and after-coming slow waves that repeat rhythmically at a rate of three cycles per second (Fig. 11.5). Each burst typically lasts between 1 and 3 seconds, but longer runs occur, especially when activated by hyperventilation (HV) or drowsiness. These bilateral bursts are synchronous in timing and symmetric in amplitude between hemispheres, although shifting asymmetry is common from burst to burst. On close inspection, latency differences between hemispheres can be detected, but usually by no more than 20 milliseconds. The amplitude of these discharges may be upward of several hundred microvolts and typically most prominent in the midline frontal region.
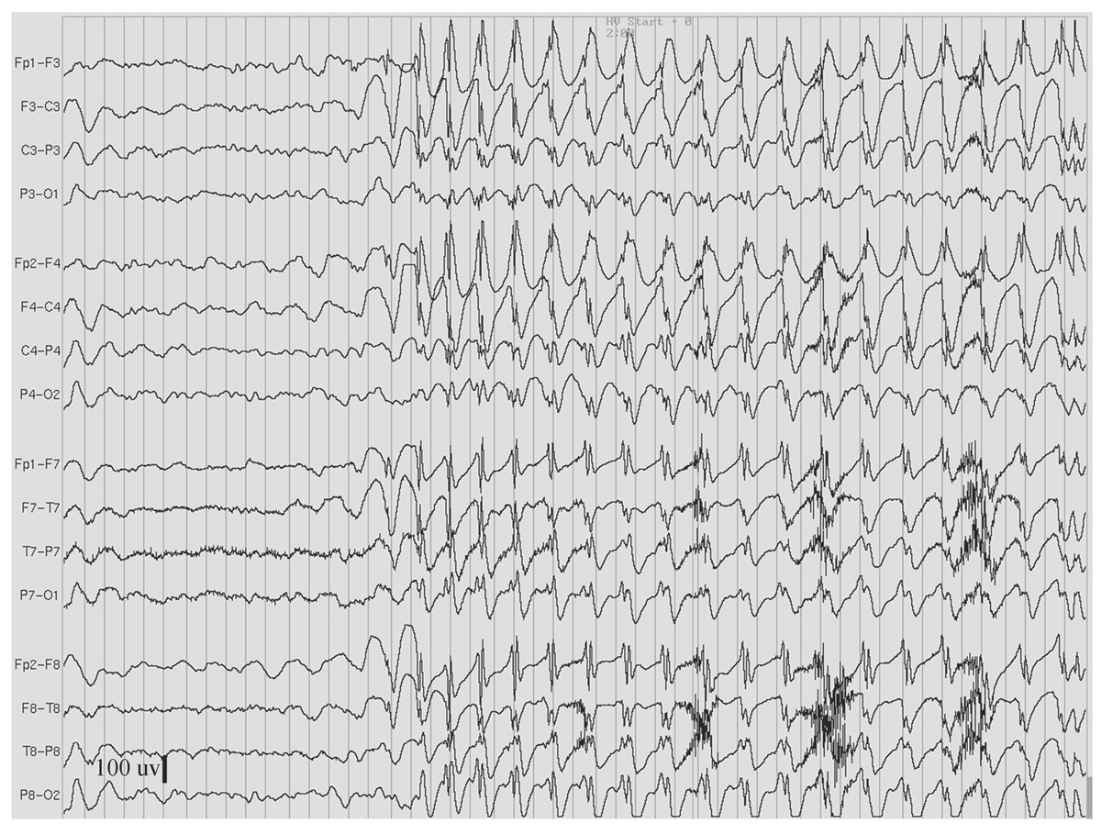
Figure 11.5: Hyperventilation-induced paroxysm of 3-Hz spike-and-waves in an 11-year-old girl who had behavioral arrest and eyelid fluttering during the paroxysm. (Interval between gridlines is 200 milliseconds.)
3-Hz spike-and-wave discharges are the EEG signature of absence epilepsy. It is now known that even a brief burst can interfere with mental functioning. The effect is subtle and may not be apparent by visual observation; however, neuropsychological testing has demonstrated that even a 1- or 2-second burst will briefly interrupt continuous motor tasks (22). Bursts of 3 seconds or longer duration are more consistently accompanied by the clinical signs of an absence seizure, namely, behavioral arrest, staring, and/or eye fluttering.
HV is a standard procedure for activating absence seizures and 3-Hz spike-and-wave bursts during EEG recording. However, clinicians must be aware of pseudo-absence events that can occur in some children without epilepsy (23). This nonepileptic phenomenon has EEG features of runs of high-voltage, semirhythmic to rhythmic, delta waves that may be slower than the usual HV buildup. This slowing is widely distributed, but often with a maximal amplitude at the anterior head regions. However, careful analysis of the EEG in pseudo-absence spells shows that spikes are lacking, unlike real 3-Hz spike wave. Unfortunately, the clinical appearance of pseudo-absence spells can mimic that of genuine absence seizures (24). Accordingly, an EEG recording is required to differentiate the two.
Generalized Atypical Spike-and-Slow-Waves
Generalized atypical spike-and-slow-wave discharges are bilaterally synchronous complexes that resemble 3-Hz spike-and-wave discharges, but differ in that they have variable rates, although mostly close to 4 Hz. Also, the spike component of the complexes is often polyphasic (25) (Fig. 11.6). Moreover, the complexes vary greatly in amplitude and morphology within and between bursts. The atypical spike-and-slow-wave pattern is more likely to occur as single bursts rather than in long repetitive runs. Drowsiness and non-REM sleep activate generalized atypical spike-and-slow-wave discharges, and they may be entirely absent during wakefulness in some patients.
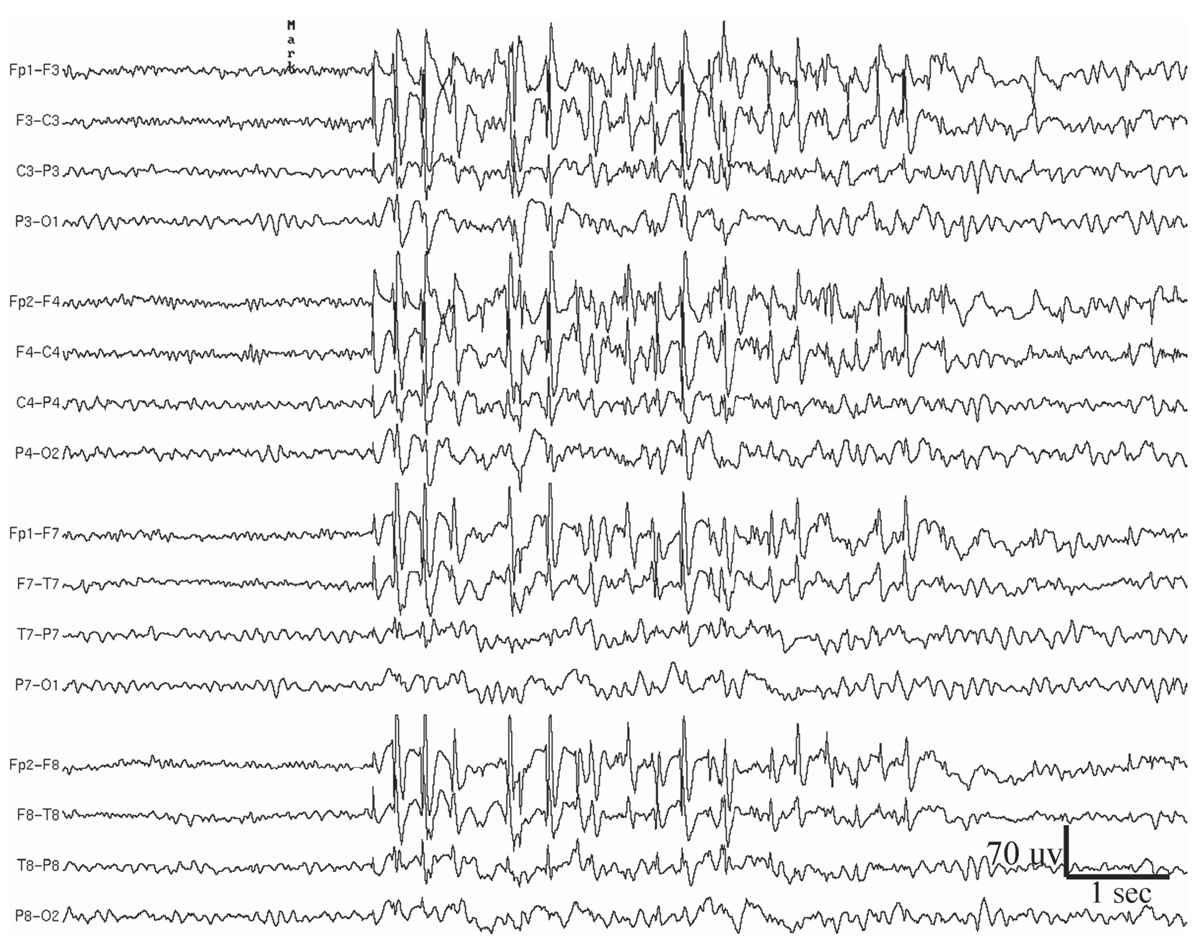
Figure 11.6: Generalized atypical spike-and-slow-wave complexes in a 19-year-old woman who since age 13 years has had sporadic generalized tonic-clonic seizures on awakening in the morning. The patient did not have myoclonic jerks or absence spells, but the recorded paroxysms, such as the one shown in this figure, were sometimes accompanied by brief arrest of activity.
The clinical correlation of the generalized atypical spike-and-slow-wave abnormality is with primary generalized epilepsy, including benign myoclonic epilepsy of early childhood, myoclonic-astatic epilepsy of early childhood, juvenile myoclonic epilepsy (JME) of Janz, juvenile absence epilepsy, atypical absence epilepsy, epilepsy with grand mal on awakening, and photosensitive epilepsy (26).
In patients with both 3-Hz and atypical spike-and-slow waves, it is not uncommon for focal spikes of low amplitude to appear during drowsiness in the frontal or temporal regions. These do not indicate an accompanying focal seizure disorder, as long as they are not abundantly present at a single location during wakefulness or sleep.
Slow Spike-and-Waves (Sharp-and-Slow-Wave Complexes)
As the name indicates, the slow spike-and-wave discharge pattern is characterized by complexes that occur slower than the “prototypical” 3-Hz spike-and-wave discharge pattern of absence epilepsy (Fig. 11.7), namely, around 1.0 to 2.5 Hz. Moreover, the complexes are not as rhythmic, and the spike component is replaced by longer-duration sharp waves (27). Compared to 3-Hz spike-and-wave pattern, persistent or fluctuating asymmetry of amplitude between hemispheres occurs more commonly with the slow spike-and-wave pattern. This pattern is also more likely to occur during waking as a single discharge of one or a few complexes. However, drowsiness or non-REM sleep may activate trains of repeating slow spike-and-wave complexes, sometimes similar to electrical status epilepticus during sleep (ESES). HV, but not photic stimulation, may enhance slow spike-and-waves, but not as reliably as it does the 3-Hz spike-and-wave pattern. The slow spike-and-wave pattern is commonly seen as one of the EEG features of Lennox-Gastaut syndrome (LGS) in children and adults.
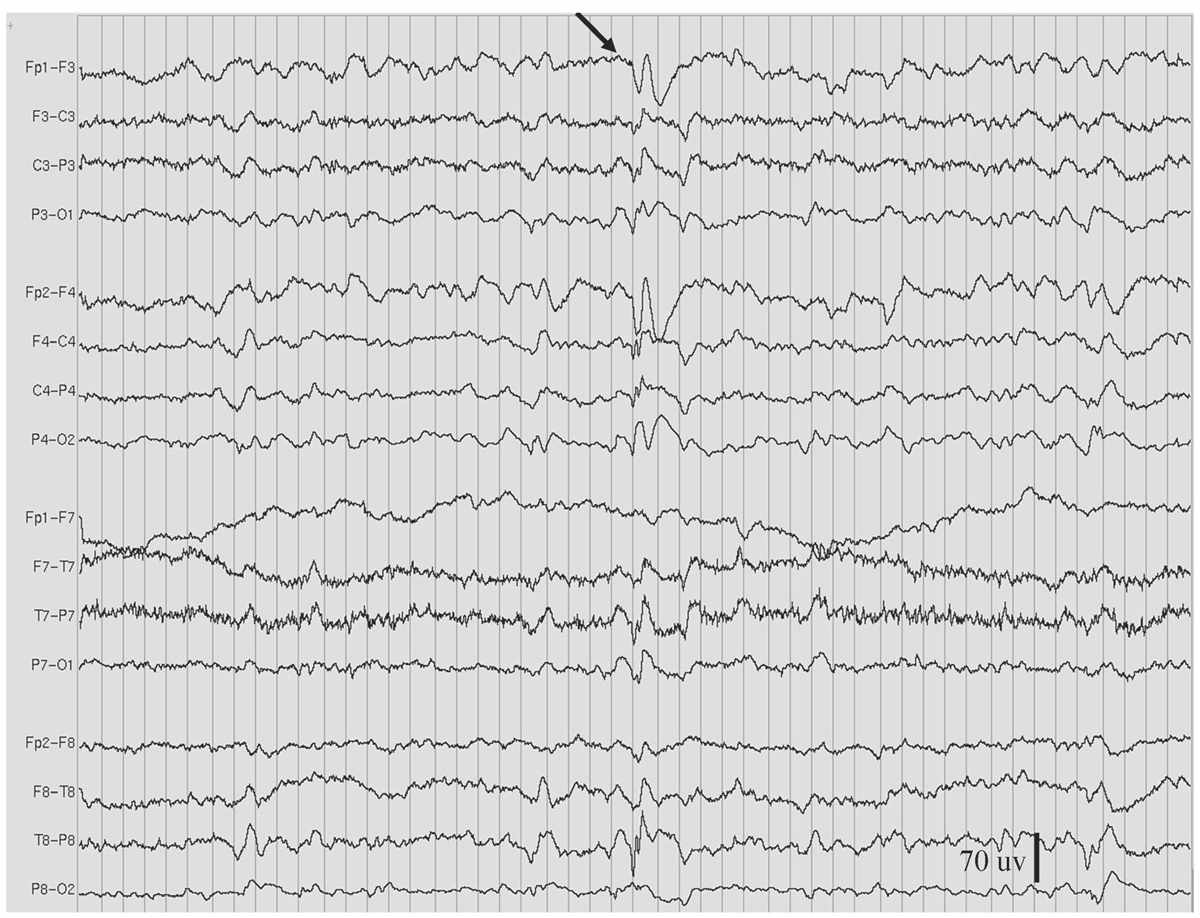
Figure 11.7: Slow spike-and-wave, also called sharp-and-slow wave (indicated by arrow), in the patient in Figs. 11.2 and 11.9. (Interval between gridlines is 200 milliseconds.)
Generalized Repetitive Fast Discharge
GRFD is also known as paroxysmal fast rhythm, generalized paroxysmal fast activity, or “runs of rapid spikes” (28,29). This pattern consists of bursts of repetitive spikes in the alpha or beta frequency range (Fig. 11.8). The bursts are generalized in distribution, typically last less than 10 seconds, and are of low to medium amplitude.
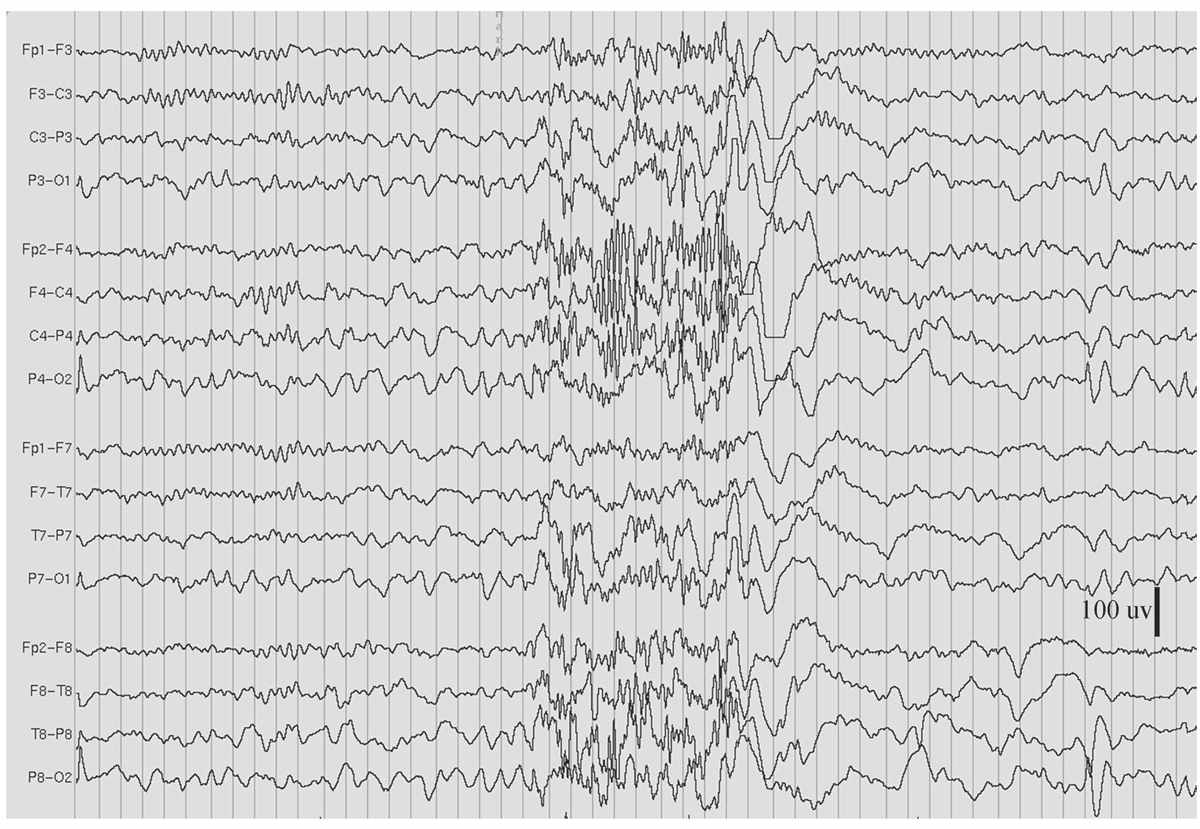
Figure 11.8: GRFD during sleep, in the patient in Figs. 11.2 and 11.7. (Interval between gridlines is 200 milliseconds.)
Most GRFD occurs during sleep. It can be considered an ictal rhythm, because tonic seizure activity sometimes accompanies it. However, this tonic seizure activity may be subtle and take the form of tonic slow eye opening. GRFD may also be accompanied by transient apnea or bradycardia in some children. A particular type of GRFD, referred to as an electrodecrement, is characterized by a sudden, brief, generalized attenuation of background EEG. High-frequency, but low-amplitude, spiking is thought to underlie electrodecremental events. Patients with GRFD usually also have generalized spike-and-wave complexes or especially slow spike-and-wave discharges. Bursts of GRFD may be preceded or followed by these other discharges. GRFD is often associated with catastrophic epilepsy syndromes, especially in children, such as the LGS (29,30).
Photo-Epileptiform Discharges (Photoparoxysmal Response)
Photo-epileptiform discharges are spikes/sharp waves or complexes of spike-and-waves that are elicited by repetitive photic stimulation. Photo-epileptiform discharges have been divided into two types—“self-limited,” if the discharges do not exceed the duration of the stimulation; and “self-sustaining,” if they outlast the stimulation (31). Self-sustaining discharges are generally believed to be more highly associated with seizure disorders; however, two studies showed no difference in seizure propensity (32,33).
Photo-epileptiform discharges can also be divided into four categories according to their scalp distribution: (a) generalized (Fig. 11.9); (b) bilateral posterior dominant (Fig. 11.10); (c) bilateral occipital; and (d) focal unilateral (34). Of the four categories, generalized photo-epileptiform discharges are the most common, and focal unilateral discharges are the least common. When focal, photo-epileptiform discharges usually occur in the occipital region, and they have a high association with a structural abnormality ipsilateral to the discharges. Up to 77% of individuals with generalized photo-epileptiform discharges have seizure disorders, whereas epilepsy is less common in those with bilateral occipital discharges. In general, primary generalized epilepsy is most commonly associated with photo-epileptiform discharges. Focal seizure disorders are rarely associated with this phenomenon, but those rare instances when focal seizures are activated by photic stimulation, they usually begin in the occipital region.
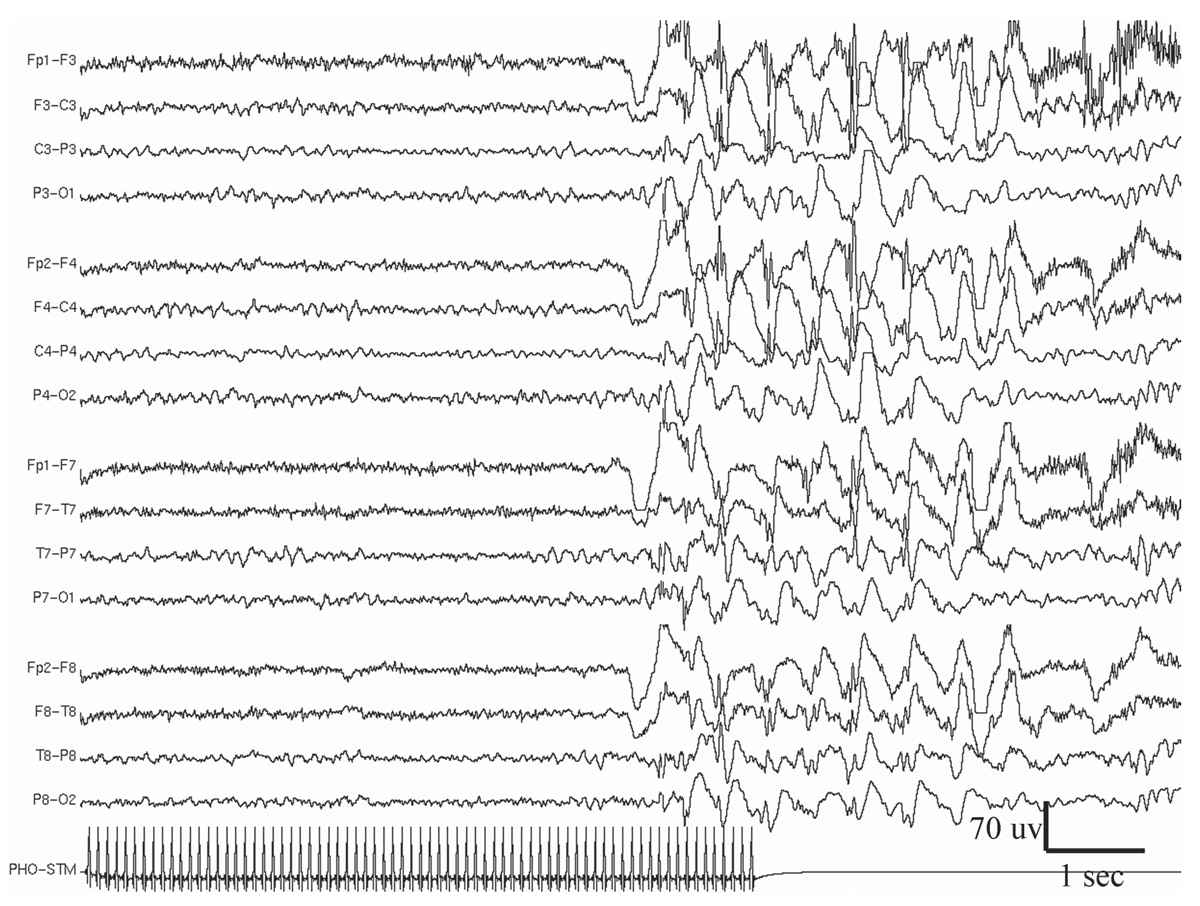
Figure 11.9: Example of generalized photo-epileptiform discharge (photoparoxysmal response).
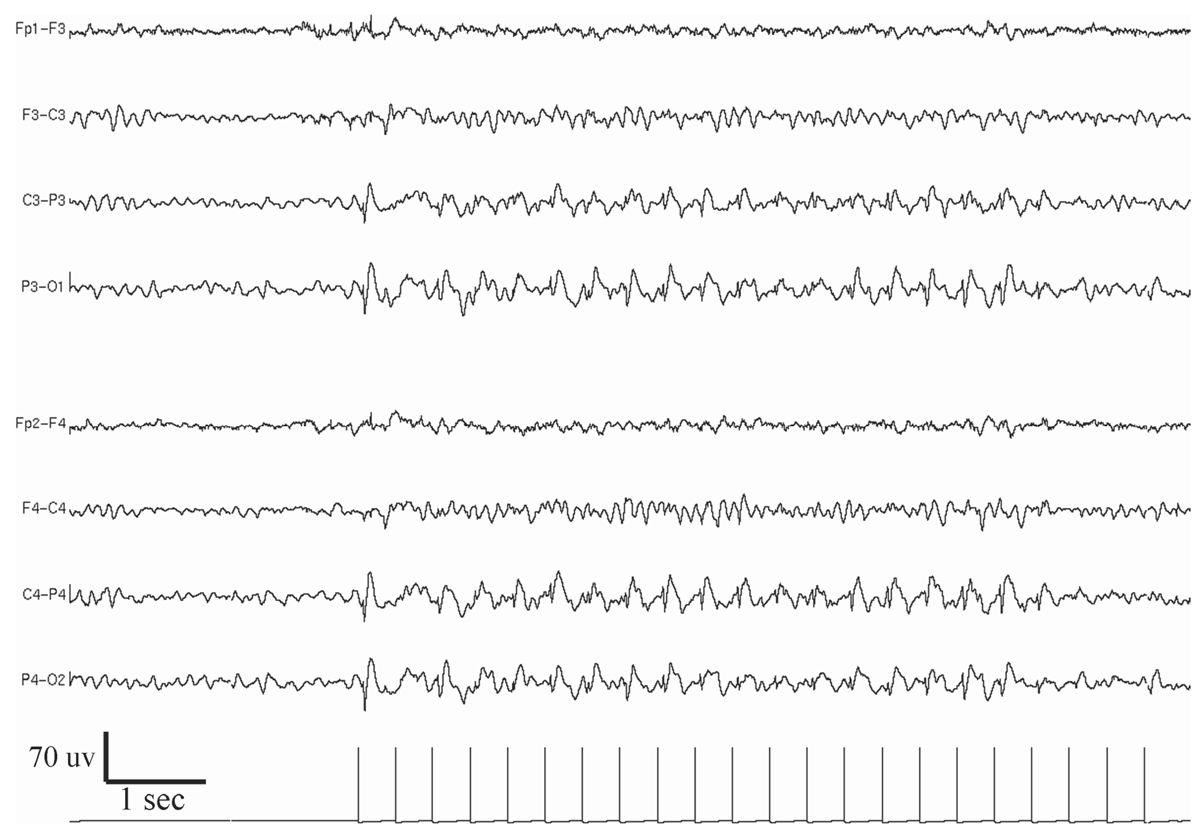
Figure 11.10: Example of bilateral posterior dominant photo-epileptiform discharge (photoparoxysmal response).
An EEG recording during a patient’s habitual episode and documenting an ictal discharge are essential parts of the evaluation of patients with paroxysmal episodes, particularly for differentiating epileptic from nonepileptic spells. In patients with epilepsy, ictal EEG recording is often needed to help determine seizure type and epilepsy syndromes. Ictal recordings are also an indispensable part of the presurgical evaluation of patients with pharmaco-resistant seizures (35).
Features of Scalp-Recorded Ictal Discharges
Seizure patterns can be isomorphic or metamorphic. Isomorphic patterns lack clear evolution between multiple phases of an ictal event. EEG morphology changes little throughout the seizure. A typical example is the spike-and-slow wave discharge of an absence seizure. On the other hand, the EEG morphology of the metamorphic seizure pattern evolves through different stages of the seizure. This pattern is most typical of focal seizures, but it can be seen in both primary and secondary generalized tonic-clonic seizures.
Defining what constitutes an ictal pattern can be difficult. It is usually, but not always, associated with seizure symptoms or signs in the patient. Ictal onset is generally marked by a clear change in the background EEG rhythm, which may be manifested as an increase or decrease of the frequency, field, morphology or amplitude of activity as the seizure evolves. Seizures are most recognizable when the EEG pattern consists of an organized rhythm that is different from normal background activity and has apiculate or sharp waveforms. Fortunately, ictal EEG patterns typically evolve through several stages, which is an important distinguishing feature.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


