Myocardial Protection
Richard J. Shemin
Yousef M. Odeh
This chapter reviews current concepts regarding myocardial protection during cardiac procedures including minimally invasive procedures with or without robotic assistance. In addition, myocardial protection in orthotropic heart transplant will be discussed. We provide the rationale of our strategy and describe the methodology of our approach in detail.
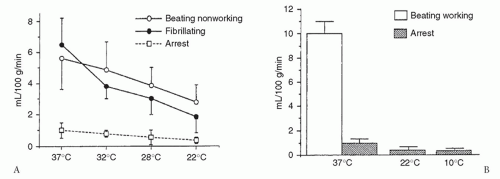
The strategies available for myocardial protection have led to adversarial positions regarding warm versus cold blood cardioplegia, as well as antegrade versus retrograde delivery. This creates confusion and potentially deprives the patient of the benefits of each method. In principle, cardioplegia markedly reduces oxygen demand in the arrested heart and must be delivered in sufficient quantity to all regions to match myocardial demand (Fig. 37.1). This has led to our use of antegrade and adjunct retrograde delivery, cold methods to reduce the demands allowing bloodless visualization, and warm reperfusion for resuscitation.
Our “integrated method” is a strategy of myocardial protection that combines the advantages of different concepts, allowing the operation to be conducted without interruptions.
The principle goals of cardioplegia are:
Providing and maintaining electromechanical arrest of the myocardium.
Sustained cooling of the myocardium, while flushing out unwanted toxins and warm blood.
Limiting myocardial edema and providing buffering capacity.
Limiting ischemic and reperfusion injury to the myocardium.
Electromechanical activity during cardiac arrest raises oxygen demand during ischemia. Hypothermia is a crucial component of myocardial protection because it reduces myocardial oxygen demand (Fig. 37.1). Blood cardioplegia is now preferred by most surgeons because of its versatility. It maintains oncotic pressure, is a natural buffering agent, has advantageous rheologic properties, and is a free-radical scavenger. Blood cardioplegia also limits reperfusion injury and reverses ischemia/reperfusion changes in the acutely ischemic myocardium. These beneficial features may not be possible with crystalloid cardioplegia.
Noncardioplegia methods have been applied in special situations. For example, when a severely calcified aorta is encountered, the preferred technique is deep hypothermic arrest (approximately 20°C) without aortic clamping.
COLD BLOOD CARDIOPLEGIA
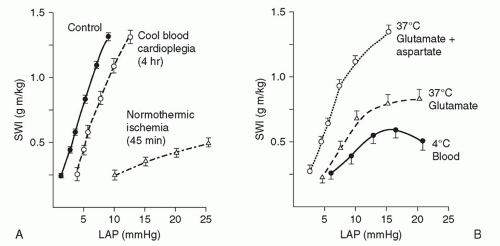
Cold blood cardioplegia allows complete myocardial recovery after 4 hours of ischemia in normal hearts. Hypothermia lowers myocardial oxygen demand and ischemic damage when coronary flow is interrupted in the course of the revascularization procedure, provided the cardioplegic perfusate is distributed adequately with reinfusions. Hypothermia alone, however, does not avoid injury in chronically “energydepleted” (ischemic) hearts (Fig. 37.2). Furthermore, hypothermic crystalloid cardioplegia adds some disadvantages, such as shifting the oxyhemoglobin association curve leftward, retarding Na+/K+ adenosine triphosphatase to produce edema activation of platelets, and creating free radicals. Blood cardioplegia consists of four parts of blood to one part of crystalloid solution. This limits the hemodilution seen with crystalloid cardioplegia during repeated infusions.
WARM BLOOD CARDIOPLEGIA
Warm blood cardioplegia (37°C) given initially (induction) limits reperfusion damage in ischemic hearts. It enhances metabolic repair by channeling aerobic adenosine triphosphate production to reparative processes. Other cardioplegic components, citrate phosphate dextrose (CPD) and buffers (THAM [tromethamine; tris-hydroxymethylaminomethane]), limit calcium influx and acidosis. Clinical studies confirm experimental findings and show that warm blood cardioplegia (“hot shot”) after ischemia improves recovery. Warm cardioplegic induction and reperfusion solutions are augmented with the amino acids glutamate and aspartate to replenish key Krebs-cycle intermediates depleted by ischemia. These additions enhance the reparative processes after a period of myocardial ischemia (Fig. 37.2).
Warm (37°C) cardioplegia may also be used after aortic unclamping to restore rhythm and improve hemodynamics in the unstable patient following cardiac surgery. When used for impaired hemodynamics, the left ventricle requires venting to reduce distention and myocardial stress. The antegrade flow is given for 5 to 10 minutes.
ANTEGRADE/RETROGRADE PERFUSION: INTERMITTENT OR SIMULTANEOUS?
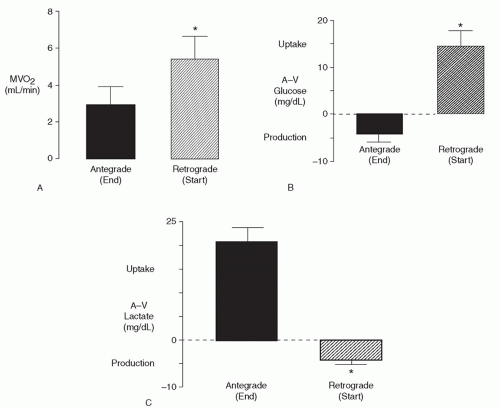
A cardioplegic perfusate requires even distribution. Adding retrograde perfusion improves subendocardial perfusion, avoids ostial cannulation during aortic valve procedures, limits removal of retractors during mitral procedures, and permits flushing of air and atheroma during coronary reoperations. Transatrial coronary sinus cannulation allows safe and rapid retroperfusion into the coronary sinus and has become widely adopted. Experimentally, right ventricular nutritive flow appears limited, thus hypothermia is essential to lower oxygen demand and provide effective right ventricular protection. Clinical studies show that switching from antegrade to retrograde perfusion increases oxygen uptake and lactate washout, indicating that each mode perfuses different areas. Therefore, both antegrade and retrograde perfusions are required (Fig. 37.3).
The limitations of antegrade and retrograde delivery were first overcome by using both antegrade and retrograde perfusions intermittently. It is possible to simultaneously deliver retrograde cardioplegia via the coronary sinus and antegrade cardioplegia via direct ostial or vein graft infusion. Myocardial venous hypertension is prevented by drainage through the Thebesian veins. Studies document the safety of simultaneous vein graft and coronary sinus perfusion.
INTERMITTENT/CONTINUOUS INFUSION
Continuous cardioplegic perfusion has been advocated to avoid ischemia by antegrade or retrograde delivery, but adequate protection may not be achieved at usual flow rates and vision becomes obscured during infusion. A quiet/dry field requires “intentional” ischemia by intermittently stopping the flow of cardioplegic solution. Intermittent replenishment restores hypothermia, maintains electromechanical arrest, flushes accumulated metabolites, and counteracts acidosis and edema. Initially, the heart is arrested with high-dose potassium (20 to 40 mEq/L) blood cardioplegia (Table 37.1), and low-dose cold potassium (8 to 10 mEq/L) blood cardioplegia (Table 37.2) is used for intermittent repeat doses. Such reinfusions (every 15 to 20 minutes) are often retrograde, directly into the aorta or, via the each coronary ostium. Cold blood with a hematocrit of
approximately 20% is infused at a pressure of <40 mmHg and infused for a duration of 2 minutes.
approximately 20% is infused at a pressure of <40 mmHg and infused for a duration of 2 minutes.
Table 37.1 Cold Blood Cardioplegia Solution | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Table 37.2 Cold Multidose Blood Cardioplegia Solution | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
At the completion of the cardiac procedure, controlled reperfusion is achieved by administering a dose of warm cardioplegia (“hot shot”), administered either antegrade, retrograde, or both. This can be followed by a continuous infusion of warm blood into the aortic root while the cross-clamp is still in place at a flow of 300 to 350 ml/min with a pressure of ≤80 mmHg. The aortic clamp is released within 5 minutes after adequate contractility is observed.
CANNULAS AND DEVICES FOR DELIVERING AND MONITORING ANTEGRADE/RETROGRADE AND SIMULTANEOUS ANTEGRADE/RETROGRADE CARDIOPLEGIA
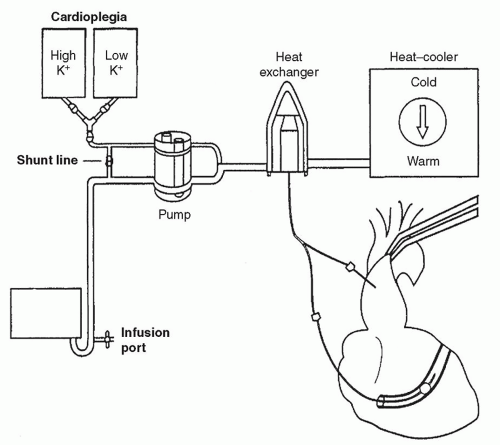
All operations include the use of a cardioplegic heat exchanger for cold and warm perfusion (Fig. 37.4) cannulas for
antegrade and retrograde delivery and a monitoring-infusion system. The system we use is shown in Figure 37.5. This has been found to be the simple, yet effective way for delivering and monitoring cardioplegia in conventional cardiac cases.
antegrade and retrograde delivery and a monitoring-infusion system. The system we use is shown in Figure 37.5. This has been found to be the simple, yet effective way for delivering and monitoring cardioplegia in conventional cardiac cases.
The main cardioplegia line is connected to a three-way stopcock. From here, the cardioplegia is set with branches allowing either antegrade flow or retrograde flow. The retrograde catheter has a port to transducer pressure allowing measurement and display of the waveform. The stopcock allows alternating between antegrade and retrograde flows.