Chapter 33 Myocardial Blood Flow Measurement
Evaluating Coronary Pathophysiology and Monitoring Therapy
INTRODUCTION
Positron emission tomography (PET) combined with tracer kinetic modeling allows the assessment of regional myocardial blood flow (MBF) of the left ventricle in mL/g/min and adds a new dimension to the noninvasive evaluation and understanding of coronary pathophysiology. Measuring coronary flow responses to sympathetic stimulation with cold pressor testing and/or hyperemic flow increases due to pharmacologic vasodilation enable the noninvasive identification and characterization of coronary circulatory function. These flow responses can identify both functional and structural abnormalities of the coronary circulation, reflecting early stages of developing coronary artery disease (CAD) long before the development of macroscopic morphologic alterations of the arterial wall and the clinical manifestation of CAD. PET measurements of MBF combined with various forms of vasomotor stress, therefore, extend the scope of conventional scintigraphic imaging in the noninvasive delineation of coronary circulatory dysfunction in early subclinical stages of CAD. The identification of the CAD process does not depend on demonstrating regional perfusion defects at rest or during stress on conventional myocardial perfusion imaging. Rather, the extent of coronary flow responses to vasomotor stress or the coronary flow reserve may help identify coronary functional abnormalities that may precede or accompany CAD-related structural alterations of the arterial wall.1
METHODOLOGY OF BLOOD FLOW MEASUREMENT
PET approaches for the measurement of regional MBF in mL/g/min entail intravenous administration of positron-emitting tracers of MBF such as nitrogen-13-labeled (13N) ammonia, oxygen-15-labeled (15O) water, or rubidium-82 (82Rb) (Table 33-1) and imaging of the radiotracer’s transit time through the central circulatory system and its extraction and retention in the myocardium. 13N-ammonia and 15O-water, the most commonly used flow tracers with PET, are short-lived (physical half-life 9.8 and 2.4 minutes, respectively) and therefore necessitate on-site cyclotron production. Rubidium-82, with an ultrashort physical half-life of 78 seconds, is available through a strontium-82/rubidium-82 generator system with a 4- to 5-week shelf life and thus does not require an on-site cyclotron.
Table 33-1 Tracers of Myocardial Blood Flow
| Tracer | Half-Life | Method |
|---|---|---|
| O-15-water | 2.4 min | Cyclotron |
| N-13-ammonia | 9.8 min | Cyclotron |
| Rubidium-82 | 78 sec | Generator |
Cyclotron, in-house cyclotron needed; Generator, available through generator; Half-Life, physical half-life.
Beginning with the intravenous injection of the radiotracer of blood flow, serial images are acquired to capture the initial transit of the flow tracer through the central circulation and its extraction into the myocardium (see Table 33-1). The serially acquired image data sets are then reformatted into short-axis and long-axis myocardial slices and assembled into polar maps.2,3 Regions of interest (ROIs) are assigned by the analysis software program to the territories of the three major coronary arteries; a 25 mm2 ROI is positioned in the left ventricular blood pool on a short-axis slice. The ROIs are then copied to all serially acquired image data sets, and time-activity curves are generated that reflect the arterial radiotracer input function and the myocardial response to it. The time-activity curves are then fitted with operational equations derived from tracer kinetic models, which describe the exchange of radiotracer between tissue compartments and the volume of tracer distribution in each compartment. The operational equations then yield regional MBF in mL/g/min. Tracer kinetic models also correct for physical decay of the radioisotope, partial volume–related underestimations of the true myocardial tissue concentrations (by assuming a uniform myocardial wall thickness of 1 cm),4 and spillover of radioactivity between the left ventricular blood pool and myocardium.5
PET TRACERS OF MYOCARDIAL BLOOD FLOW (See Chapter 19)
Estimates of MBF obtained with 13N-ammonia and 15O-water have been widely validated against independent microsphere blood flow measurements in animals and yielded highly reproducible values of MBF (correlation coefficient for 13N-ammonia: r = 0.99 and standard error of the estimate (SEE): 0.17; correlation coefficient for 15O-water: r = 0.95).6,7 Similarly, measurements of MBF with 13N-ammonia and 15O-water in humans yield comparable estimates of MBF,8,9 but these tracers differ in several aspects in their use to measure MBF.
13N-Ammonia
Used as a myocardial flow tracer, 13N-ammonia is selectively retained by the myocardial cells and has a longer physical half-life (9.8 minutes). This allows acquisition of statistically high-count 13N-ammonia images of the myocardium, and thus the relative distribution of the myocardial flow can be denoted with high diagnostic quality in the visual and semiquantitative assessment of myocardial perfusion defects during stress underlying hemodynamically obstructive CAD.10–15 Although 13N-ammonia becomes metabolically trapped in myocardium, mainly in the form of glutamate and mostly glutamine, alterations in cardiac work or myocardial metabolism do not significantly affect the association between tracer tissue concentrations and MBF.10 Acute ischemia may moderately reduce the retention fraction of 13N-ammonia in the myocardium, but it does not significantly affect its use as a flow tracer. The longer physical half-life of 13N-ammonia, however, necessitates longer time intervals (≈45 minutes) between repeat assessments of MBF, restraining the numbers of interventions that can be undertaken during a single PET flow study.
Rubidium-82
As a myocardial flow tracer, 82Rb is increasingly used for the assessment of myocardial perfusion or its relative radiotracer uptake of the left ventricle during stress and rest in the evaluation of flow-limiting epicardial stenosis.12,16–22 Recent investigations also suggest the utility of 82Rb for quantifying MBF in mL/g/min.23–27 The ultrashort physical half-life of 82Rb (only 76 seconds) allows serial investigations of myocardial perfusion at short time intervals (e.g., 10 minutes). Yet the ultrashort physical half-life necessitates the delivery of high 82Rb activity doses (i.e., 60 mCi) for adequate visualization of the myocardial tracer uptake after 1 to 2 physical half-lives for tracer clearance from the blood pool. Clinical investigations report the feasibility and utility of MBF quantification with 82Rb PET combined with one- or two-compartment tracer kinetic models.24–27 Initial animal validation studies24,28 using a two-compartment tracer kinetic model yielded precise MBF values at rest, while the hyperemic flow was generally underestimated and highly variable. Applying 82Rb with a one-compartment tracer kinetic model, however, appears to be more promising in the quantifications of MBF.26,27 In 14 healthy volunteers, for example, estimates of MBF both at rest and during dipyridamole-stimulated hyperemia correlated well with flow values obtained with 13N-ammonia (r = 0.85). Mean hyperemic MBF values were comparable between both flow tracers (13N-ammonia: 2.71 mL/g/min versus 82Rb: 2.83 mL/g/min); however, the corresponding standard deviations (SD), as an index for the individual measurement variability between both flow studies, was higher for rubidium-82 with 0.81 mL/g/min when compared to 13N-ammonia with an SD of 0.50 mL/g/min.27 In another investigation,25 PET measurements of myocardial blood flow with 82Rb using a wavelet-based noise-reduction protocol29 and a two-compartment model were compared to myocardial flows as determined with 15O-water in eleven healthy individuals. For these two approaches to quantify myocardial flow, the authors found a close association of resting and hyperemic MBF (r = 0.94; P < 0.001) ranging between 0.45mL/g/min to 2.75 mL/g/min, emphasizing the potential role of 82Rb in the quantification of myocardial blood flows by PET. Further studies on the interstudy and interobserver variability of 82Rb-estimated MBF are needed to determine the exact range of measurement-related errors of this approach in the individual person and, thereby, its utility in the assessment of subclinical and clinically manifest CAD.
15O-Water
As a myocardial flow tracer, 15O-water distributes into the water spaces of both the myocardium and the blood pool. Thus, 15O-water does not selectively accumulate in the myocardial cells, and corrections for the blood-pool activity are needed. These corrections are commonly performed by blood-pool imaging with 15O water or 11C-labeled red blood cells and subtraction of the blood pool from the 15O water images. Applying a one-compartment tracer kinetic model, estimates of MBF are then determined from the rate of clearance of 15O-water from the left ventricular myocardium.7,30 The subtraction of the blood pool from the 15O water images, in concert with the rapid clearance of 15O-water and its short half-life, may produce statistically low-count images of the myocardium. Thus, the visual evaluation and semiquantitative analysis of the relative distribution of the myocardial 15O-water uptake (“static” image) may be of limited value in the diagnostic evaluation of flow-limiting epicardial lesions. On the other hand, using 15O-water, with its short physical half-life (2.4 minutes), affords repeat MBF measurements at short intervals of 10 to 15 minutes. This allows a convenient assessment of MBF at rest as well as the response to vasomotor stress before and after intervention during the same study session.
DETERMINANTS OF MYOCARDIAL BLOOD FLOW AND FLOW RESERVE
Coronary Circulatory Function: Definitions
The investigation of coronary vasomotor (circulatory) function is usually confined to patients with chest pain syndromes undergoing coronary angiography. Although the identification of coronary vasomotor dysfunction during coronary angiography entails important diagnostic and prognostic information,31–36 the invasive nature and time-consuming process to determine this abnormality in vasomotor function pose major limitations. Invasive approaches to assess abnormalities in coronary vasomotor function, therefore, cannot be applied for widespread clinical use.37–43 A variety of invasive methods for evaluating coronary vasomotor function exist.37,44,45 Such methods commonly necessitate computer-based measurements of coronary vessel diameter (QCA; quantitative coronary angiography) and intracoronary flow-velocity probes for determining coronary flow alterations in response to infused acetylcholine, bradykinin, or substance P–stimulated release of endothelium-derived nitric oxide (NO), or flow-mediated (and thus endothelium-dependent) alterations of the lumen of the epicardial artery.37,38,42,44,45 As regards the latter approach, increases in coronary flow can be stimulated either by intracoronary infusion of papaverine or adenosine or by a more physiologic stress test such as sympathetic stimulation with cold pressor testing (CPT).36–38,40,46
In more detail, endothelium-dependent vasodilators such as acetylcholine specifically stimulate the muscarinic receptor–mediated release of endothelium-derived NO that induces the epicardial artery to dilate through relaxation of the vascular smooth muscle cells. It is worth noting that such vasodilation in response to acetylcholine can only be partially prevented by inhibitors of endothelial NO synthase (eNOS).37 Thus there appears to also be a concomitant release of other endothelium-derived vasodilators, such as prostacyclin and/or endothelium-derived hyperpolarizing factors (EDHF) that contribute to the acetylcholine-stimulated normal coronary vasodilation.37,45 The specific intracoronary infusion of acetylcholine, for example, through an infusion catheter defines normal endothelial function when there is a vasodilation of the epicardial artery. In the presence of dysfunctional epicardial artery endothelium, however, the concurrent smooth muscle cell constrictor effects of acetylcholine overcome the endothelium-mediated vasodilation,37 and a lack of vasodilation or, more commonly, a paradoxical vasoconstriction ensues.37,47 For the determination of coronary blood flow to evaluate the responses of the coronary microcirculatory system, an intracoronary Doppler catheter and the placement of a flow wire are necessary.37,45 Endothelium-dependent vasodilators such as acetylcholine also increase coronary blood flow, paralleling their concurrent vasodilator effects on the coronary arteriolar resistance vessels. Increases in coronary flows to acetylcholine stimulation as measured with the Doppler flow wire, therefore, identify normal endothelium-dependent coronary arteriolar vasomotor function, whereas an impairment or absence of coronary flow is appreciated as endothelial dysfunction of the arteriolar vessels.37,48 Notably, a similar phenomenon has been described for sympathetically induced coronary flow increases during CPT with immersion of the left hand into ice water.40,46,48,49 Normally, CPT-induced and metabolically induced vasodilation of the coronary arteriolar vessels induces an increase in flow in the coronary circulatory system. This increase in coronary flow results in an elevation of shear forces on the vascular wall, with a concomitant release of endothelial-derived NO50,51 and thereby induces a flow-mediated vasodilation of the upstream coronary vessels.37,38,46 If the endothelium is dysfunctional, however, the sympathetically mediated vasoconstrictor response of the vascular smooth muscle cells during CPT prevails and causes an impairment, absence, or even decrease in coronary flow.46,52–54 Of note, the CPT-induced coronary vasomotor response closely correlates with the specific determination of coronary vasomotion due to acetylcholine stimulation at the site of the epicardial conduit artery and the coronary microcirculation.40,48,52,55
Substances such as nitroglycerin or sodium nitroprusside provide NO directly to the vascular smooth muscle cell layer, causing epicardial vasodilation. Thus, the latter vasomotor response to nitroglycerin or sodium nitroprusside is independent of the functional state of the vascular endothelium and thereby provides specific information on vascular smooth muscle cell function of the epicardial artery.37 Similarly, adenosine or papaverine relax vascular smooth muscle cells of the coronary vessels and are commonly applied intracoronarily to gain information on the predominantly endothelium-independent hyperemic coronary flows or so-called total coronary vasodilatory capacity. At the same time, determination of flow-mediated epicardial vasodilation by QCA during hyperemic flow increases with adenosine or papaverine may serve as another important and more physiologic index of endothelium-dependent epicardial vasomotion.37,40,56 Applying such a protocol, a flow-related and NO-mediated epicardial vasodilation during a hyperemic coronary flow increase defines normal endothelial function, whereas an impairment or absence of flow-related epicardial vasodilation is indicative of a dysfunctional state of the coronary endothelium.
NONINVASIVE ASSESSMENT OF CORONARY CIRCULATORY FUNCTION
Myocardial Blood Flow at Rest
PET-measured MBF at rest in healthy volunteers has been reported to range between 0.4 and 1.2 mL/g/min.23,57–61 Apart from some interstudy variations of PET-measured MBF related to methodologic differences, including radiotracers, tracer kinetic models, and image analysis, the variability in these individual resting blood flows is likely attributable to differences in left ventricular myocardial workload at the time of assessment.57,62 As observed in several clinical investigations,57,58,63 there is a close linear correlation between resting MBF and the rate-pressure product (RPP; defined as the product of systolic blood pressure and heart rate) as an index of cardiac work and therefore metabolic oxygen demand. These observations indicate that increases in myocardial work are closely accompanied by commensurate flow increases to adequately meet increases in oxygen demand. Accordingly, MBF is closely coupled to myocardial oxygen demand and myocardial work, both at rest and during physical stress (e.g., bicycle exercise, treadmill exercise, dobutamine stimulation).14,63 Age-related increase in resting MBF has also been related to increases in cardiac work due to higher systolic blood pressures.58,61 More controversial are possible differences in MBF at rest between males and females. Some but not all investigations observed higher values of resting MBF,57,61,64 that are thought to be related to gender-dependent differences in plasma lipid profiles.
Assessment of Coronary Reactivity
Approaches to assessing coronary circulatory function include measurements of MBF with PET at rest and its responses to physiologically or pharmacologically stimulated coronary flow increases, including bicycle exercise, dobutamine stress, sympathetic stimulation with CPT, as well as vascular smooth muscle relaxation with vasodilator agents.14,46,63,65–71 An additional indicator of coronary function is a heterogeneous perfusion response to pharmacologic or sympathetic stimulation, with a progressive decrease in perfusion from the base to the apex of the left ventricle. This abnormal perfusion response might prove useful as a noninvasive indicator of epicardial coronary dysfunction.72,73
Total Integrated Vasodilator Capacity and Coronary Flow Reserve
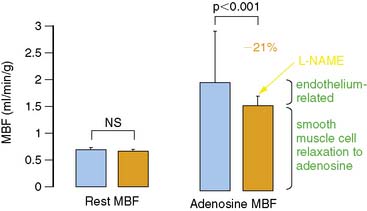
The most widely and clinically used approach for the assessment of coronary circulatory function is the pharmacologically induced hyperemic MBF.62,65,70,74,75 Vascular smooth muscle–relaxing substances such as dipyridamole, adenosine, adenosine triphosphate (ATP), or adenosine receptor agonists76 lower the resistance to flow at the site of the coronary arterioles and produce maximum or submaximum hyperemic MBF. Hyperemic flow is considered a measure of a predominantly endothelium-independent flow response. However, inhibition of eNOS by intravenous infusion of NG-nitro-l-arginine methyl ester (L-NAME) significantly reduces adenosine-induced MBF increases by 21% to 25%, as measured with PET (Fig. 33-1).77,78 This attenuation in hyperemic flow responses most likely reflects an impairment of flow-mediated vasodilation during higher coronary flows. Thus, shear-sensitive components of the coronary endothelium contribute, in part, through flow-mediated coronary vasodilation to the overall hyperemic flow during pharmacologic vasodilation.37,77,78 Since pharmacologically induced hyperemic MBF increases reflect smooth muscle cell and, in part, endothelium-related vasodilatory effects, it is also defined as the “total integrated coronary circulatory function.”44,65,70
The coronary vasodilatory capacity reflects the ability of the coronary circulatory system to increase flows from baseline in order to meet increases in myocardial metabolic demand. This capacity to increase coronary flow to a maximum from rest is known as coronary flow reserve and first described by Coffman and Gregg.79 A more physiologic framework for the coronary flow reserve was provided by Mosher et al.80 by adding the concept of coronary autoregulation. Within the latter concept, resting coronary blood flow is determined by several factors, while myocardial oxygen demand—as a function of heart rate, blood pressure, myocardial contractility, and ventricular preload—is appreciated as the predominant factor in the regulation of resting coronary flows. Given that the metabolic myocardial oxygen demand is widely constant, coronary flow within the range of its autoregulation is widely independent of the coronary perfusion pressure. Consequently, within the range of coronary autoregulation, so-called plateau coronary flow changes little, despite alterations in perfusion pressures. Conversely, a pharmacologically induced dilation of the coronary arteriolar resistance vessels leads to a hyperemic coronary inflow that no longer is governed by autoregulatory mechanisms and that changes linearly with changes in intracoronary perfusion pressure. In this consideration, the ratio of hyperemic to resting coronary flow is defined as the coronary flow reserve. Values for the flow reserve should be interpreted with caution, however. Under certain conditions, the coronary flow reserve does not necessarily indicate the true coronary vasodilator capacity. The coronary flow reserve can decline due to increase in resting flow or decrease in maximum hyperemic flow. Factors that increase the demand for myocardial oxygen, such as arterial hypertension, increased myocardial contractility, increased left ventricular wall stress, and tachycardia, cause an increase in resting flow. On the other hand, maximum hyperemic coronary flow may decline in the presence of a focal flow-limiting epicardial lesion, in the presence of microvascular disease in patients with hypertension or diabetes, or as a consequence of increases in extravascular resistive forces paralleled by increases in left ventricular pressures in patients with congestive heart failure or hypertension. Thus, there are several limitations when interpreting the coronary flow reserve. Nevertheless, the concept of the coronary flow reserve remains an important and useful index for assessing the functional significance or downstream effects of focal epicardial arterial lesions, functional improvement after coronary revascularization, and coronary circulatory function in individuals with subclinical or clinically manifest CAD.65,81,82
Sympathetic Stimulation with Cold Pressor Testing
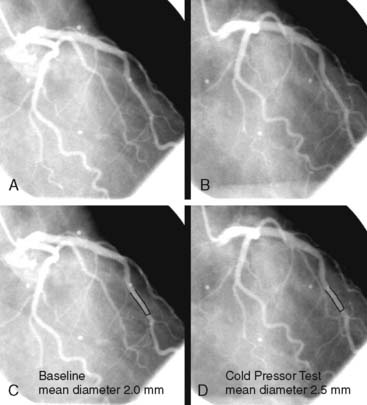
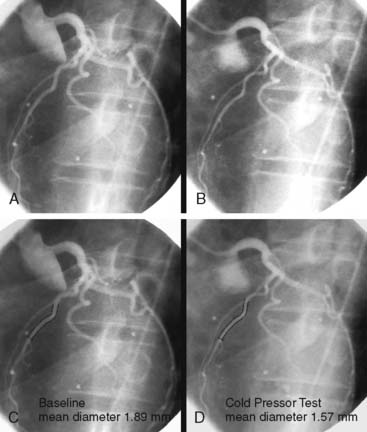
PET measurements of alterations in MBF from rest to sympathetic stimulation by CPT entail specific information on coronary endothelial function.39,46,48,53,83,84 CPT is performed with immersion of a hand into ice water, which in turn induces a sympathetically mediated increase in heart rate and blood pressure and thus an increase in myocardial workload. The RPP can be used as an index of myocardial workload and oxygen demand. Increases in myocardial workload during CPT are associated with vasodilation of the coronary arteriolar resistance vessels through the release of (presumably) adenosine as a metabolic vasodilator.85 As a consequence, there is a decrease in coronary vascular resistance that causes an increase in coronary inflow. This increase in coronary inflow causes in turn a flow-mediated, endothelium-dependent dilation of the upstream coronary vessel segments. As demonstrated in Figure 33-2, an increase in cardiac work is normally accompanied by commensurate flow-mediated coronary vasodilation, and an increase in MBF is determined with PET.46,53,54,61 In the presence of coronary endothelial dysfunction, however, the sympathetically induced increase in coronary inflow does not mediate a flow-related vasodilation. Under such conditions, the sympathetically mediated vasoconstrictor effects of the vascular smooth muscle cells predominate and are not overcome by normal flow-related coronary vasodilation (Fig. 33-3).37,46,86 Myocardial blood flows during CPT are then attenuated, absent, or even paradoxically decreased, which denotes a dysfunction of the coronary endothelium.46,53,54,84
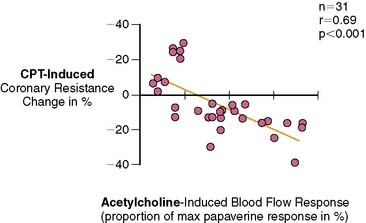
Several clinical investigations in the assessment of coronary vasomotor function emphasize the validity and value of PET-measured changes in MBF during CPT from rest as a noninvasive index of coronary endothelial function. For example, coronary flow increases during CPT, as assessed invasively with Doppler wire during coronary angiography, closely correlate with the flow response to acetylcholine stimulation, which is considered a specific probe of endothelial function (Fig. 33-4).37,48 Coronary flows during sympathetic stimulation with CPT, therefore, may probe endothelium-related vasomotor function and thereby delineate the functional integrity of the vascular wall.37,46 In particular, it was observed that the epicardial vasomotor response to CPT was closely paralleled by the acetylcholine-stimulated response, suggesting the epicardial vasomotor response to CPT may be intimately related to the integrity of endothelial function.37,52 Further, CPT-induced changes in epicardial luminal diameter, as determined by QCA, and PET-measured responses of MBF to CPT, are closely correlated.46,53,54 More direct evidence for the involvement of the endothelium in CPT-mediated MBF responses has been provided by Campisi et al.84 In chronic smokers exhibiting an impairment of MBF responses to CPT, intravenous infusion of l-arginine as a substrate of nitric oxide synthase restored the MBF increase to CPT, most likely due to increases in the bioavailability of endothelium-derived NO.
Perfusion Heterogeneity and Base-to-Apex Myocardial Perfusion Gradient
In the last 2 decades, the interrelation of structural and functional determinants of clinically manifest CAD has been extensively studied.87–92 As shown by previous fundamental work of Gould et al.,87 focal coronary artery lesions between 60% and 85% diameter stenosis commonly do not affect the resting MBF, as the result of a compensatory vasodilation of the downstream arteriolar resistance vessels.87 This adaptive vasodilation under resting conditions may fail to compensate for greater increases in epicardial resistance, that is, for cases of severe coronary artery stenoses (greater than 85%), so stress-induced myocardial ischemia may manifest.69,93 In light of several investigations, it has been widely accepted that hyperemic coronary flows during pharmacologic vasodilation commonly begin to decline when focal epicardial artery narrowing reaches 50% diameter stenosis.87,88,90,92
Whether structural changes of the arterial wall in early stages of CAD may also affect the coronary flow is an area of ongoing debate and interest. PET flow studies1,82,94–96 add evidence that hyperemic flow increases may be mildly diminished in the presence of diffuse CAD with coronary lesions less than 50% and/or coronary circulatory dysfunction. Such observations have raised a new concept that even subclinical CAD-related structural alterations of the arterial wall may exert downstream fluid dynamic effects that, as reported recently, may manifest as mild myocardial perfusion heterogeneity at rest,97,98 and during vasomotor stress98 or as longitudinal base-to-apex perfusion gradient during hemodynamic stress.72,73,94,99 Gould et al.94 were the first to describe heterogeneity in longitudinal myocardial perfusion during pharmacologically induced hyperemia in patients with diffuse CAD. Other investigators found that PET-determined quantitative estimates of MBF during pharmacologically induced hyperemia also denoted a relative decrease in longitudinal myocardial flow from the mid to the mid-distal part of the left ventricular myocardium in individuals with coronary risk factors but without clinically manifest CAD.72,73 It has been argued that fluid dynamic consequences of CAD- related vessel stiffness and/or functional abnormalities of the epicardial conduit vessels may account for the longitudinal heterogeneity in myocardial perfusion. Based on the Hagen-Poiseuille equation, intracoronary resistance relates to the velocity of the blood flow and inversely to the fourth power of the vessel diameter.91,100,101 Normal function of the vascular endothelium ascertains that increases in flow velocity during exercise or pharmacologic vasodilation are associated with a flow-dependent and nitric-oxide-mediated dilation of the coronary artery, which balances the velocity-induced increase in coronary resistance, so that the resistance is kept low or does not increase.38,42 Structural and/or functional disturbances of coronary arterial walls in the early development of CAD, however, commonly impair a flow-mediated and thus endothelial-derived NO–mediated dilation of the epicardial artery. In this concept, the absence of a flow-mediated epicardial vasodilation causes an increase in intracoronary resistance during higher coronary flows that leads to a progressive proximal-to-distal decline in intracoronary pressure along the epicardial artery.100 Such a decrease in intracoronary pressure has been put forth as cause for a gradual base-to-apex, relative decline or heterogeneity in myocardial perfusion or MBF.72,73,94,100
There is some direct evidence of a cause-and-effect relationship of structural and/or functional changes of the epicardial artery and downstream hemodynamic effects. As previous investigations have demonstrated, there is a close association between heterogeneity in myocardial perfusion during dipyridamole stimulation and the presence of diffuse CAD.94 A more recently performed comparative study of patients with coronary risk factors and angina pectoris symptoms provided further insight.102 Comparisons were made between the invasive assessment of epicardial endothelial function in response to acetylcholine stimulation, and 99mTc tetrofosmin SPECT-measured myocardial perfusion during both bicycle exercise and dipyridamole stimulation. In these patients without flow-limiting epicardial coronary lesions, a moderate but significant association (r = 0.49; P < 0.002) between acetylcholine-induced alterations in epicardial artery diameter and the degree of exercise-induced myocardial perfusion defects was observed, suggesting that functional abnormalities of the epicardial artery may indeed account, at least in part, for stress-induced regional myocardial perfusion defects.102,103 A perfusion heterogeneity in longitudinal (base-to-apex) MBF during sympathetic stimulation with CPT, as measured with PET, was observed to be related to a functional decrease in epicardial luminal diameter as determined with quantitative coronary angiography (r = 0.77; P < 0.0001).99 Apart from structural alterations of the coronary arterial wall, an impairment of flow-mediated, endothelium-dependent epicardial coronary vasomotor function may contribute to the manifestation of a longitudinal heterogeneity in MBF during vasomotor stress.44,103 The assessment of heterogeneity in longitudinal myocardial perfusion or quantitative blood flow with PET could be a promising noninvasive index of early structural and/or functional alterations of the CAD process, predominantly at the site of the epicardial artery. As another noninvasive index of the early stages of the development of CAD, it may carry important predictive information on future cardiovascular events95,104 but awaits further confirmation through clinical endpoint investigations.
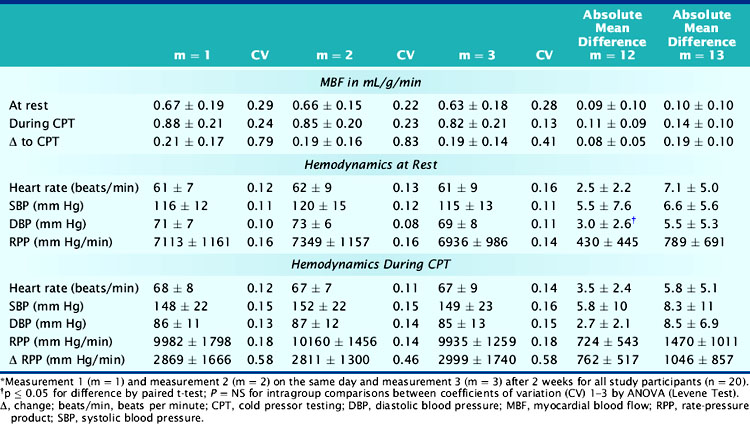
Reproducibility of Measurements of Myocardial Blood Flow and Responses to Stressors
As outlined before, an impairment of coronary circulatory function in individuals with subclinical stages of the CAD process has been widely appreciated to carry important diagnostic and prognostic information.31,32,105–107 If this holds true, then a restoration of abnormalities in coronary circulatory function by lifestyle modifications or therapeutic interventions should lead to an improved clinical outcome, as some preliminary data of the peripheral circulation may indeed suggest.108–110 PET measurements of MBF responses to cold exposure and pharmacologic vasodilation are increasingly applied to determine and monitor the effects of lifestyle modifications or therapeutic interventions on coronary circulatory function.53,67,111–117 Such serial PET flow studies raise the need for establishing the reproducibility of repeat PET measurements of MBF.118 This allows determination of the methodologic measurement error of PET-determined flows in repeat assessments62,74,118 and also the biological and hemodynamic variability of these flow measurements.46,58,118–120 By using the mean difference of repeat MBF measurements, the sample size of the study populations needed for adequately powering clinical investigations with serial PET blood-flow measurements can be determined.
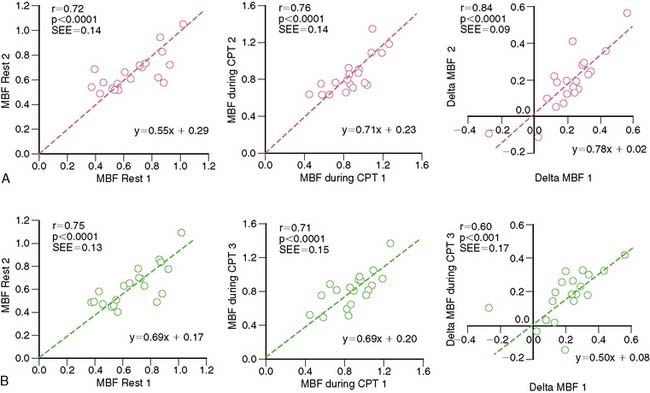
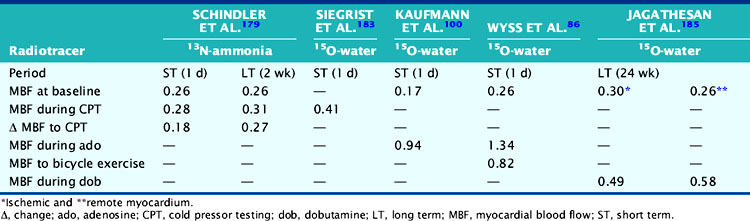
A reasonable reproducibility of hyperemic MBF during pharmacologic vasodilation with 13N-ammonia or 15O-labeled water and PET has been demonstrated previously.66,74,121 As recently shown, CPT-related MBF measurements were also reproducible when determined with 15O-labeled water on a 1-day protocol.122 Further, in a more detailed and extended investigation,118 the hemodynamic and endothelium-related MBF responses to CPT or its change from rest (ΔMBF), as measured with 13N-ammonia and PET, were demonstrated to be not only highly reproducible in short-term (1-day protocol) but also in long-term (2 to 3 weeks protocol) measurements (Table 33-2). There are important considerations in interpreting the reproducibility data of PET-measured MBF. For example, the Pearson’s correlation coefficient (r) of the least-square regression analysis can be used to denote the strength of agreement between repeat MBF measurements. Notably, the standard error of the estimate (SEE) indicates the tightness of the linear fit of the data and is considered to represent the actual range of method-related measurement error (or its variability). For example, the range of measurement errors, as denoted by the SEE for the endothelium-related change of MBF from rest to CPT, was found to be 0.09 mL/g/min for short-term and 0.17 mL/g/min for long-term repeat measurements (Fig. 33-5). According to the latter values of the SEE, alterations in the change in MBF in serial pharmaceutical studies that are above this range of SEE are likely to be related to beneficial effects of pharmaceutical interventions on coronary endothelial function.118 In addition, the mean difference and the corresponding standard deviation (SD) of repeat MBF measurements are used to calculate the sample size of a study population needed to sufficiently power a serial flow study. For example, using the longitudinal mean difference and the corresponding SD of the endothelium-related change in MBF from rest to CPT of 0.08 ± 0.05 mL/g/min in the short-term and of 0.19 ± 0.10 mL/g/min in the long-term (see Table 33-2), at a 5% significance level with a power of 87%, a sample size of 14 and 22 individuals would be needed for serial PET flow studies to identify possible intervention-related, statistically significant alterations in MBF responses to CPT.118 Another important statistical parameter used in the interpretation of reproducibility data is the repeatability coefficient (RPC) as proposed by Bland and Altman.123 The RPC serves as a useful index to denote the agreement between repeat measurements. Given a normal Gaussian distribution of MBF measurements,123 the RPC denotes the expected range of measurement error between repeat assessments of myocardial blood flows. It is critical, however, to keep in mind that most PET flow studies have investigated relatively small sample sizes, between 11 and 25 study participants.66,74,118,124 Given the relatively small sample sizes of these PET flow studies, they may not have fully met the assumption of a normal Gaussian distribution of flow values. Further, the RPC can also be applied as an index of precision of MBF measurements between different studies (Table 33-3). For example, in the aforementioned investigation,118 the RPC for the endothelium-related change in MBF from rest to CPT was 0.18 mL/g/min for the short-term and 0.27 mL/g/min for the long-term reproducibility measurements with 13N-ammonia and PET. Both RPCs indicate less measurement error in the assessment of endothelium-related MBF responses to CPT with 13N-ammonia PET than was observed with the short-term RPC of CPT-related flows and 15O-water measurements (1-day study protocol)122 and also for hyperemic flow increases reported in previous studies,66,74,124 which have been reported between 0.49 and 1.34 mL/g/min (see Table 33-3).
ALTERED CORONARY CIRCULATORY FUNCTION AND CARDIOVASCULAR EVENTS
Normal endothelium-dependent vasomotor function has been widely appreciated to play an active and central role in the modulation of the integrity and metabolism of the vascular wall, vasomotor tone, and hemostasis.125,126 The coronary vasomotor tone underlies the endothelium-dependent production and release of vasoactive mediators such as prostacyclin, endothelin-1, endothelium-derived hyperpolarizing factor (EDHF), and in particular, NO. Given normal function of the coronary endothelium, increases in coronary flow with physical exercise lead to a flow-mediated and thus endothelium-dependent release of NO, prompting a NO-mediated relaxation of the vascular smooth muscle cells and vasodilation. This NO-mediated mechanism offsets possible vasoconstrictor effects of elevated endothelin-1 and angiotensin-II levels, and/or sympathetic activation.37,44,127
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree