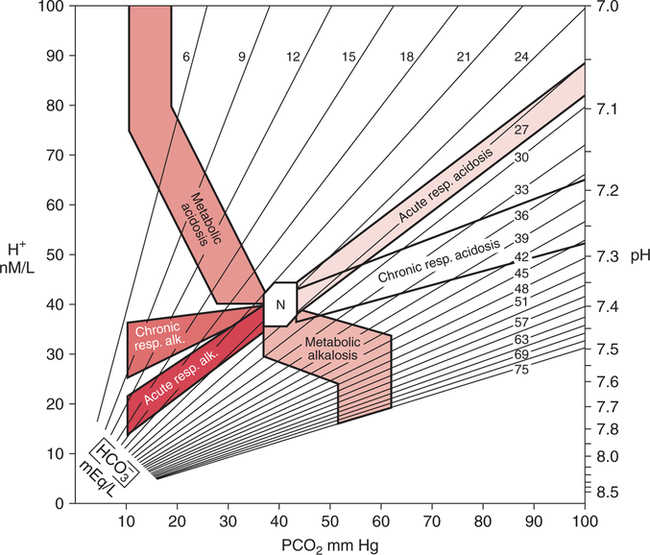
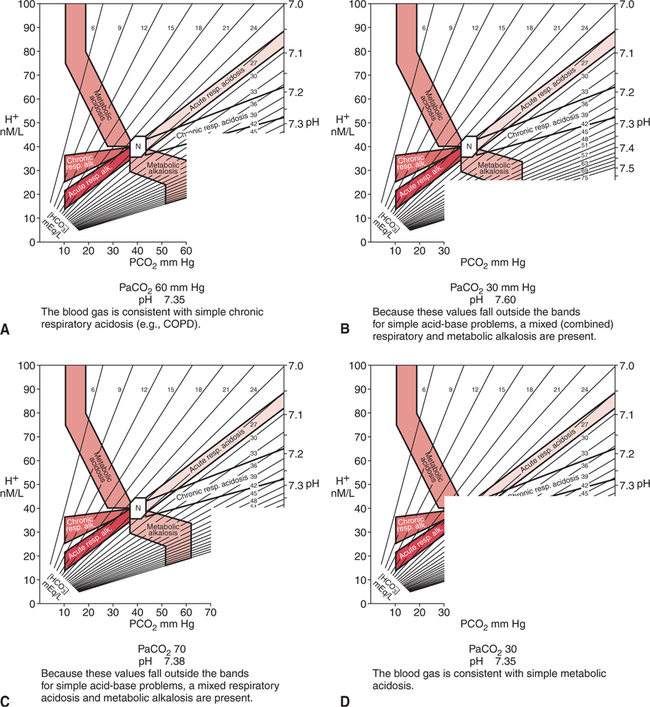
Typical End-Stage COPD Blood Gases The pH is often within the normal range (i.e., completely compensated) and may even be on the alkalotic side of the normal range.10 This finding is not consistent with rules that apply to compensation (i.e., overcompensation should not occur), however, and may be related to a mild concurrent primary metabolic alkalosis. The administration of steroids and diuretics with concomitant hypochloremia or hypokalemia is common in severe COPD. Many patients with severe COPD respond paradoxically to acute hypoxemia or oxygen therapy in that their PaCO2 increases instead of decreases. Reasons for this are unclear but are most likely related to worsening ventilation-perfusion mismatch and exhaustion secondary to the work of breathing. In addition, as described previously, excessive oxygen therapy may similarly precipitate acute hypercarbia. This affect has also recently been shown to occur during acute asthma exacerbations and the administration of FIO2 1.0.700 Excessive oxygen therapy may also be recognized by the concurrent presence of a PaO2 in excess of 60 mm Hg. When acute hypercapnia is superimposed on typical COPD chronic blood gases, the result may appear as shown in Example 14-4. Patients with COPD who present with blood gas results such as those shown in Example 14-4 can often be treated successfully with low concentrations of oxygen therapy, noninvasive ventilation, and bronchial hygiene.543 544 Thus, mechanical ventilation, with related discomfort and the potential for complications, can often be avoided. Furthermore, a blood gas such as this may be the first clue that the patient has COPD. This finding, in turn, alerts the clinician to the potential for increasing hypercapnia with excessive oxygen therapy. Therefore, recognition of this situation may have great clinical importance. The natural tendency of the body to compensate for primary acid-base disturbances was discussed in Chapter 8. Because of this natural phenomenon, whenever opposing respiratory and metabolic conditions were present, compensation was assumed. Although this initial assumption is logical, it may be incorrect. It is not uncommon to have two opposing primary acid- base disturbances that give the surface appearance of simple compensation. The coexistence of two primary acid-base disturbances is called a mixed acid-base disturbance. How can simple compensation be differentiated from a mixed acid-base disturbance? Probably the most useful aid in this regard is the acid-base map that is shown in Figure 14-1. The labeled areas encompass with 95% confidence the range of pH, PaCO2, and bicarbonate that one would expect to find in patients who have only one simple acid-base disturbance. Separate bands are also given for both acute and chronic acid-base problems. Figure 14-2 shows how the acid-base map can be used by simply aligning the two adjacent sides of a piece of paper with the patient’s respective PaCO2 (horizontal axis) and pH (vertical axis). The corner point of the paper represents where the values intersect on the map. The data in Figure 14-2,A are consistent with chronic simple respiratory acidosis. Remember, this does not mean that the elevated bicarbonate cannot be due to a primary problem, but only that the data are consistent with usual compensation for chronic respiratory acidosis. Figure 14-2,B does not fall within any band, therefore the clinician can be relatively certain that there are two separate, primary, acid-base disturbances (respiratory alkalosis and metabolic alkalosis). Figure 14-2,C similarly represents two primary acid-base disturbances, although in this case they are in opposite directions (i.e., respiratory acidosis and metabolic alkalosis). Without an acid-base map, one might assume that these blood gas results are due to complete compensation. Finally, Figure 14-2,D is consistent with a simple metabolic acidosis. Table 14-1 shows the typical compensatory response to respiratory acidosis. This table shows that when the PaCO2 increases to 70 mm Hg acutely, the pH drops immediately to approximately 7.22 (0.06 decrease/10 mm Hg PaCO2 increase). The immediate increase in bicarbonate is a result of the hydrolysis effect that was discussed in Chapter 8 and it does not represent renal compensation. Table 14-1 *In comparing metabolic acidosis and alkalosis, note that to produce the same acute change in pH (∼0.2 unit), a much larger change in [HCO3−] is necessary in metabolic alkalosis (ΔHCO3−= 16 mEq/L) than in metabolic acidosis (ΔHCO3− = 9 mEq/L). Modified from Kokko, J.P., and Tennen, R.L.: Fluids and Electrolytes, Philadelphia, W. B. Saunders, 1986, p. 386. Compensation for respiratory alkalosis is similar in magnitude to compensation for respiratory acidosis. In general, the pH should return at least halfway back toward normal. Again, an example is shown in Table 14-1. Surprisingly, however, when the respiratory alkalosis persists for weeks, the pH may actually return completely to normal in some cases.515 Renal compensation for respiratory alkalosis requires the excretion of bicarbonate; therefore, hyperchloremia often develops to preserve electroneutrality. The major portion of the ventilatory response to metabolic acidosis usually begins quickly; however, the maximal compensatory response may take up to 24 hours.545 546 When metabolic acidosis develops in the plasma, it takes some time for the pH to fall in the cerebrospinal fluid owing to the limited permeability of ions across the blood-brain barrier. Lactic acidosis, however, may actually develop within the brain cells, and it is therefore associated with a more rapid ventilatory response.515 A very useful rule of thumb when an acid-base map is not at hand is that after maximal compensation, the PaCO2 generally approximates the last two digits of the pH.515 Thus, in simple chronic metabolic acidosis with a pH of 7.30, the PaCO2 is usually approximately 30 mm Hg (see Table 14-1). The respiratory compensatory response to metabolic alkalosis is hypoventilation with retention of carbonic acid. It has long been assumed, however, that this response was limited by the onset of hypoxemia. Therefore, it is often stated that compensation for metabolic alkalosis will not allow the PaCO2 to exceed 55 to 60 mm Hg.515 More recent reviews have shown that hypoventilation is not limited by hypoxemia.547 Progressive, linear hypoventilation accompanies progressive, simple, metabolic alkalosis when it is not associated with other acid-base problems.547 As shown in Table 14-1, compensation may sometimes also allow the pH to return halfway back to normal; however, a lesser compensatory response is more common. Therefore, the clinician must look for clues that suggest the presence of multiple (mixed) acid-base disorders. Box 14-1 suggests some situations that should alert the clinician to the likelihood of a mixed acid-base disturbance. In one report, the absence of compensation in ketoacidosis of diabetes mellitus served to alert the clinicians to the presence of a primary respiratory problem.548 The patients who did not compensate (display hypocapnia) had occult mucous plugging of major bronchi.548 After this problem was corrected, these individuals responded appropriately.
Mixed Acid-Base Disturbances and Treatment
FACTORS THAT MAY COMPLICATE CLINICAL ACID-BASE DATA
Respiratory/Renal Pathology
Chronic Obstructive Pulmonary Disease
Example 14-1
pH
7.38
PaCO2
55 mm Hg
[BE]
5 mEq/L
[HCO3]
31 mEq/L
PaO2
55 mm Hg
Acute Hypercapnia
MIXED ACID-BASE DISTURBANCES
Definition
Recognition of Mixed Disturbances
Acid-Base Map
Compensatory Patterns
Respiratory Acidosis
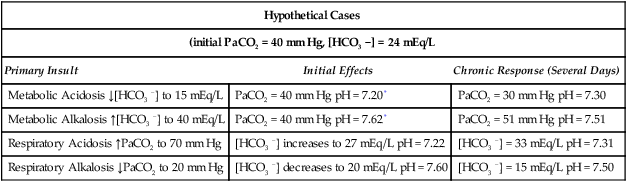
Hypothetical Cases
(initial PaCO2 = 40 mm Hg, [HCO3 −] = 24 mEq/L
Primary Insult
Initial Effects
Chronic Response (Several Days)
Metabolic Acidosis ↓[HCO3 −] to 15 mEq/L
PaCO2 = 40 mm Hg pH = 7.20*
PaCO2 = 30 mm Hg pH = 7.30
Metabolic Alkalosis ↑[HCO3 −] to 40 mEq/L
PaCO2 = 40 mm Hg pH = 7.62*
PaCO2 = 51 mm Hg pH = 7.51
Respiratory Acidosis ↑PaCO2 to 70 mm Hg
[HCO3 −] increases to 27 mEq/L pH = 7.22
[HCO3 −] = 33 mEq/L pH = 7.31
Respiratory Alkalosis ↓PaCO2 to 20 mm Hg
[HCO3 −] decreases to 20 mEq/L pH = 7.60
[HCO3 −] = 15 mEq/L pH = 7.50
Respiratory Alkalosis
Metabolic Acidosis
Metabolic Alkalosis
Alerts to Mixed Disturbances
Absence of Compensation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mixed Acid-Base Disturbances and Treatment
Only gold members can continue reading. Log In or Register to continue