Mitral Valve Repair—Robotic Minimally Invasive
Alan P. Kypson
L. Wiley Nifong
W. Randolph Chitwood
INTRODUCTION
Bailey (1951), Harken (1953), Davila (1954), and Glover (1956) made early attempts to repair mitral valves. These methods are usually related to epicardial reduction of the mitral annulus size or reducing a regurgitant orifice by “plugging” with a coneshaped prosthesis. Later, Kay (1961), Woller (1962), and Reed (1965) developed intracardiac suture annuloplasties to reduce the posterior mitral annulus length, thereby improving leaflet coaptation. McGoon (1960) performed the first repairs for ruptured chordae tendineae, and Austin (1965) first replaced a mitral valve for a papillary muscle rupture. The advent of mechanical valves impeded the progress of mitral valve repair surgery until the early 1970s, when Carpentier and Duran pioneered novel reconstructive techniques. Despite mitral valve repair successes in Europe, skepticism pervaded U.S. surgeons until Carpentier showed in 1983 excellent longterm repair results. These outcomes were based on his elucidation of the functional anatomy in insufficient mitral valves and the development of reproducible repair methods. By the late 1980s, numerous published reports from other centers validated the long-term durability of repairs, freedom from anticoagulation, and decreased mortality compared with mitral replacements.
Mitral valve surgery has been performed traditionally through a median sternotomy, which provides generous operative exposure and global cardiac access. However, during the past 15 years, improvements in instruments and endoscopes, as well as patient demand, have resulted in a substantial increase in minimally invasive general and subspecialty surgical procedures. Endovascular port-access technology first enabled less invasive surgical approaches to mitral valve surgery that avoided a median sternotomy. Advances in closed-chest cardiopulmonary bypass and myocardial protection, as well as intracardiac visualization, instrumentation, and robotic telemanipulation all have hastened a shift toward efficient and safe minimally endoscopic cardiac surgery. Today, mitral valve surgery, done through small incisions using robotic assistance, has become standard practice for an increasing number of cardiac surgeons and centers.
ROBOTIC TECHNOLOGY
Computer-assisted robotic cardiac surgery platforms have been developed to facilitate surgeon hand motions in limited operating spaces. These devices offer advantages of improved access, magnified vision, and stabilized instrument implementation. Six degrees of freedom are required to allow any free orientation of instruments in any space. Endoscopic instrumentation with four degrees of freedom reduces the dexterity significantly that is needed for delicate cardiac surgical procedures. Furthermore, the loss of depth perception with two-dimensional video monitors further increases operative difficulty. Computerenhanced (robotic) instrumentation systems overcome these and other limitations. In fact, their impact has been so great that a paradigm shift for both the patient and the surgeon has changed dramatically.
Robotic systems consist of telemanipulators with end-effectors, or microinstruments, being controlled remotely from a console. The da VinciTMS system (Intuitive Surgical, Mountain View, CA) is composed of a surgeon console, an instrument cart with four integrated arms, and a visioning platform (Fig. 43.1A and 43.1B).The operative console is removed physically from the patient and allows the surgeon to sit comfortably, immersed into the operative field using high-definition three-dimensional imaging. Digital images are translated to analog natural depth perception with high-power magnification (10×). Finger and wrist movements are registered through sensors and translated into motion-scaled tremor-free movements that avoid the fulcrum effect and instrument shaft shear forces, which are common to endoscopic instrumentation. Wrist-like articulations at the ends of microinstruments bring the pivoting action to the plane of the operative field, improving dexterity in tight spaces and allowing truly ambidextrous suture placement. A clutching mechanism enables constant readjustment of surgeon hand positions that are necessary to maintain an optimal ergonomic attitude with respect to the visual field.
EVOLUTION OF MINIMALLY INVASIVE MITRAL VALVE SURGERY
Initially, minimally invasive mitral valve surgery was based on modifications of previously used incisions and performed under direct vision. Minimal access incisions provided adequate direct vision mitral valve exposure, and surgeons showed that mitral valve operations could be done as safely and precisely as done through a larger incision. Large series by Cohn and Cosgrove showed initially that surgical mortality (1% to 3%) and morbidity were comparable to those of conventional mitral surgery. By employing familiar approaches and relying on direct vision, the initial steps in minimally invasive mitral surgery became less daunting.
With acquired experience, a shift from direct vision to video-assistance occurred and operations began to be performed using secondary vision. In 1996, Carpentier performed the first video-assisted mitral valve repair through a right minithoracotomy using ventricular fibrillation. In 1997, Chitwood described the first mitral valve replacement using videoscopic vision. The operation was done using a 6-cm right anterior mini-thoracotomy, peripheral cardiopulmonary bypass, and a percutaneous transthoracic aortic cross-clamp, and retrograde cardioplegia. In 1998, Mohr reported the Leipzig Heart Center experience of 51 minimally invasive mitral operations done using port-access technology,
a 4-cm incision, and three-dimensional videoscopy. In these patients, video technology was helpful for replacements and simple repair operations; however, complex reconstructions were still approached under direct vision. Concurrently, our group at East Carolina University reported 31 patients operated using video assistance with a two-dimensional 5-mm camera. Complex repairs, including quadrangular resections, sliding valvuloplasties, and chordal replacements, were done with no major complications and the operative mortality was <1%.
a 4-cm incision, and three-dimensional videoscopy. In these patients, video technology was helpful for replacements and simple repair operations; however, complex reconstructions were still approached under direct vision. Concurrently, our group at East Carolina University reported 31 patients operated using video assistance with a two-dimensional 5-mm camera. Complex repairs, including quadrangular resections, sliding valvuloplasties, and chordal replacements, were done with no major complications and the operative mortality was <1%.
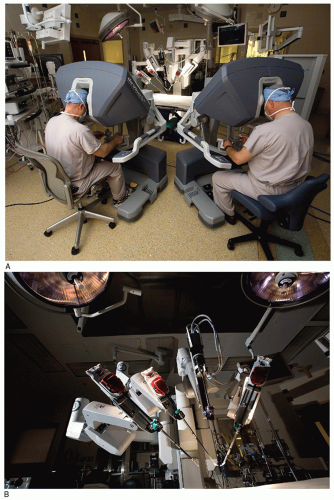 Fig. 43.1. (A) da VinciTM S Robotic Tele-manipulation System: The operative console where the surgeon is seated. (B) da VinciTM S Robotic Tele-manipulation System: The instrument cart with four arms. |
In the late 1990s, innovations in computerassisted telemanipulation occurred rapidly. In 1998, Carpentier performed the first truly endoscopic mitral valve repair using an early prototype of the da VinciTM surgical system. Within a week, Mohr performed five mitral repairs with the same device. In May 2000, our group performed the first complete mitral valve repair in North America using the da VinciTM system. In that operation, a large P2 trapezoidal resection was done with the defect closed using multiple interrupted sutures, followed by implantation of an annuloplasty band. Robotic technology has progressed to a point where at our center totally endoscopic mitral procedures have become routine for patients with isolated mitral valve pathology.
PATIENT SELECTION
All patients with isolated degenerative mitral valve disease are considered for a robotic mitral repair. Complex mitral disease is always approached with the techniques described herein. Contraindications include a prior right thoracotomy and circumferential annular calcification (Table 43.1). Moderate annular calcification alone does not preclude patients from undergoing a minimally invasive approach. However, extensive “bar” calcification remains for us a definite contraindication.
Patients with poor lung function undergo pulmonary testing to ascertain whether they will tolerate single-lung ventilation. Should patients not be able to tolerate isolated lung ventilation, cardiopulmonary bypass is instituted earlier for intrathoracic access. The transesophageal echocardiogram (TEE) remains the gold standard for perioperative planning. It is important to correlate the dynamic echocardiographic anatomy with both the Carpentier functional class and the intraoperative pathology. In patients over 40 years old, and/or with a strong family history and/or symptoms of coronary disease angiography is done preoperatively.
Table 43.1 Robotic Mitral Surgery Exclusion Criteria | |
|---|---|
|
ROBOTIC MITRAL VALVE SURGERY
Anesthesia and Monitoring
To provide single-lung ventilation, either a dual lumen endotracheal tube or bronchial blocker is used. Hemodynamic monitoring is done with a radial arterial line and a pulmonary artery catheter. For superior vena caval (SVC) drainage the right jugular vein is cannulated percutaneously with either a 15F or 17F thin-walled BiomedicusTM (Medtronic, Inc., Minneapolis, MN) arterial cannula using the Seldinger guide-wire technique. This is performed before surgical draping by our anesthesiology colleagues. Three-dimensional TEE is used to evaluate cannula placement, annular size and quality, the leaflet coaptation plane, leaflet length, and the subvalvular mitral apparatus. The intensity and direction of regurgitant jets help us to determine the significance of leaflet pathology. The transgastric view is very helpful for defining precisely which mitral segments need correction. Currently, we select the annuloplasty band size based on TEE measurements of the anterior leaflet length, annular diameter, and septal thickness. Patient-side surgeon comfort and direct vision are most important when gaining ideal intracardiac access for the placement of the robotic instrument arms and the camera. Table-side surgeon long focal-length loupes (3.5×) are most helpful for early preparation as well as passing sutures and specimens through the 3-cm incision during a da VinciTM repair.
Operative Techniques and Technology
Patients are prepared with the right thorax elevated 30 degrees on a towel roll and the right arm secured by the side. A 3-cm mini-thoracotomy incision is made in the sub-mammary fold in the anterior axillary line. After right lung deflation, the pectorals muscle is spared, and the fourth intercostal space is entered. Pericardial exposure is obtained without rib resection or division, and a soft tissue retractor (Applied Medical Resources Corp., Rancho Santa Margarita, CA) is inserted to displace the skin, fat, and muscle layers, leaving an oval space between the ribs. If the right hemi-diaphragm obstructs the operative field, a suture is placed in the central tendon and pulled through the chest wall with a “crochet hook” instrument, retracted, and secured. The pericardium is opened 2 cm anterior to the phrenic nerve and extended toward the inferior vena caval (IVC) and aortic reflections. Transthoracic pericardial retraction sutures should be placed near the SVC reflection and the atrio-IVC juncture to distract the posterior (dorsal) pericardial edge laterally. To minimize intracardiac air retention, a 14 G catheter is placed through the chest wall for continuous carbon dioxide insufflation (2 to 3 L/min). Instrument arm trocars are then placed through the third and fifth intercostal spaces and sighted to be in direct line with the mitral annulus (Fig. 43.2). It is important to maintain at least 8 to 10 cm between these arms to avoid intracardiac instrument collisions during the repair.
For cardiopulmonary cannulation, an oblique 2 cm right groin incision is made and superficial purse-string sutures are placed in both the femoral artery and vein. Then, coaxial dilators are introduced over a guide wire. Under TEE guidance, a 22F femoral vein venous cannula (Estech, San Ramon, CA) is positioned in the central right atrium. This cannula should not be suture-anchored, as intraoperative manipulation may be needed to provide optimal venous return. If SVC drainage is not possible because of patient small body habitus, a single 23/25F venous cannula (Estech) should be positioned in the SVC. By the same method, the femoral artery is cannulated with either a 17F or 19F BiomedicusTM arterial cannula. Using (kinetic-assisted) suction venous drainage, patients are cooled to 28°C systemically. Thereafter, an ascending aortic antegrade cardioplegia/vent catheter is placed just distal to the right coronary ostium. This position allows deployment of the transthoracic clamp without crowding the cardioplegia/vent catheter. Retrograde coronary sinus catheters also can be placed either across the right atrial wall or from the jugular vein and positioned using TEE.
The interatrial (Waterston’s, Sondergaard’s) groove is dissected minimally and the oblique sinus is opened behind the IVC. To occlude the aorta under either endoscopic or direct visual direction, the transthoracic aortic clamp (Scanlan, Inc., Minneapolis, MN) is placed through the second interspace in the mid-axillary line with the posterior fixed-tine directed through the transverse sinus, passing posterior (dorsal) to the ascending aorta. Care must be taken to avoid injury to the right pulmonary artery, the left atrial appendage, or the left main coronary artery. After the clamp is applied, the heart is arrested either with antegrade and/or retrograde cold blood cardioplegia.
A short left atriotomy is made inferior to the right superior pulmonary vein with extension toward and inferiorly behind the IVC. The arm of a percutaneous left atrial retractor is positioned just lateral to the sternal edge and medial to the incision, avoiding internal mammary vessels (Fig. 43.2). The dynamic retractor blade is inserted into the left atriotomy. Ventral retraction should elevate the interatrial septum to provide optimal mitral valve exposure. A left superior pulmonary vein sump sucker scavenges residual left atrial blood. Instrument arms are then passed through trocars and positioned in the left atrium, and the three-dimensional endoscope is positioned through the superior part of the incision. Valve function is evaluated using cold saline injections. The tableside surgeon is responsible for exchanging the various microtipped instruments. Standard reconstructive methods have been used in all of our da VinciTM mitral valve repairs (Table 43.2). We are comfortable in performing leaflet resections, sliding-plasties, chordal transfers, chordal replacements, as well as annuloplasties. We consider patients with Barlow’s bileaflet disease as optimal for robotic repairs.
MITRAL VALVE DISEASE AND REPAIR TECHNIQUES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


