Mitral Valve Repair in Children
Christian Brizard
INTRODUCTION/BACKGROUND
Mitral valve repair in children is guided by the same surgical rules than in adults but the anatomical substrate differs greatly. They have been set by Carpentier more than two decades ago. The technical difficulties vary according to the anatomy and to the size and age of the patient. The indications for surgery and the timing of the surgery have to take into account a large number of issues and are, therefore, more complex than in adults.
This chapter will cover mitral valve repair in children, congenital and acquired, excluding the mitral valve in atrioventricular discordance, the mitral valve in univentricular hearts, and the mitral valve of the hypoplastic left heart syndrome. We have added in this edition the surgical approach to the repair of the residual or recurrent regurgitation the left atrioventricular valve in complete atrioventricular septal defects (AVSDs).
ANATOMY AND EMBRYOLOGY
Anatomy
The normal anatomy of the mitral valve in children does not differ from the adult one and has been described. Precise knowledge of the normal anatomy is essential to read the echocardiographic study, to understand the pathological anatomy and to plan the repair.
Embryology
The leaflet and chordal tissue derive from the endocardial cushion tissue lying on the inner surface of the atrioventricular junction. The anterior leaflet originates from the superior and inferior cushions, whereas the posterior leaflet derives from an infolding of the lateral wall. As the cushion tissue elongates and grows toward the ventricular cavity, the leaflets shape progressively into a funnel-like structure totally attached to the myocardium while perforations appear in the valve leaflet edges. The perforations grow and form the chordae tendineae. The ventricular layer of the cushions will generate the fibrous part of the mitral valve and the chordae. Simultaneously, the development of the papillary muscle takes place: The anterior and posterior parts of a horseshoe ridge within the left ventricle lose contact progressively with the ventricular wall. They will form the papillary muscles, increasing their size while keeping contact with the cushion tissue at their tip.
PATHOLOGY
Congenital Anomalies of the Mitral Valve
Congenital valve stenosis and congenital mitral valve insufficiency are presented together because they have identical pathology and associated lesions. They are frequently associated in the same patient and require similar surgical techniques for the treatment.
Cleft Mitral Valve
Very often isolated, the cleft mitral valve can be easily differentiated from a left atrioventricular valve in a partial AVSD. The cleft is centered on the aortic commissure between the noncoronary cusp and the left coronary cusp, and there is no suspension apparatus on the edges of the defect. The papillary muscles are normal. Lack of valvular tissue can be seen and is secondary to the regurgitation through the cleft. The defect is not stenotic and may generate only little regurgitation for a long time.
Accessory Valve Tissue and Valvular Tags
In these anomalies, often found in association with other valvar anomalies, the spaces between the chordae are filled with a network of myxoid, valve-like tissue. When there is continuity between the anterior and the posterior leaflet, the accessory tissue may generate a gradient directly related to the size of the perforations in the accessory tissue. When the accessory valve tissue is entrapped in the left ventricular outflow tract, the mitral valve may become regurgitant due to the traction exerted by the accessory valvular tissue on the anterior leaflet, opening the valve in mid systole; however, in such cases, the left ventricular outflow tract obstruction is the predominant hemodynamic lesion and is the most frequent mode of diagnosis. The accessory mitral valve tissue in isolation often does not generate significant gradient or insufficiency.
Lesions Associated with Lack of Valvular Tissue
Three major anatomical types have been identified, although there is a continuum between them. Their recognition is useful for the planning of the repair. The functional lesion can be either predominantly regurgitant or predominantly stenotic, it can be both stenotic and regurgitant, or the valve can have a normal function.
Parachute Mitral Valve
The parachute mitral valve can be found in isolation. It can be integrated in a Shone syndrome. There is a dominant papillary muscle with the orifice of the mitral valve overriding the tip of the papillary muscle. There is a spectrum of lesions for the chordae ranging from complete absence and fusion of the tip of the papillary muscle to the free leaflet edge to relatively normal looking chordae with good mobility of the leaflet. An accessory papillary muscle, usually very small is devoted to a short segment of the free edge, or even to the under surface of the leaflet tissue with or without second orifice (double-orifice mitral valve). The functional anatomy depends on the interaction between the amount and mobility of leaflet tissue, size of the fenestrations and the presence, length, and quality of the chordae. The parachute mitral valve has almost always a stenotic component.
Papillary Muscle to Commissure Fusion
This syndrome is a spectrum. It ranges from papillary muscle tip fused to the
commissural area of the free edge to short, almost normal looking chordae. This anomaly can be limited to one papillary muscle only. The valve is generally more regurgitant than stenotic. When the papillary muscles are hypertrophied, the bulk of their mass is responsible for a valve predominantly stenotic.
commissural area of the free edge to short, almost normal looking chordae. This anomaly can be limited to one papillary muscle only. The valve is generally more regurgitant than stenotic. When the papillary muscles are hypertrophied, the bulk of their mass is responsible for a valve predominantly stenotic.
Hammock Valve (Arcade Valve)
The suspension apparatus may have lost all resemblance to the normal anatomy. There is either no papillary muscle identifiable or multiple very small ones behind the posterior leaflet. The leaflets are suspended directly by a network of chordae directly attached to the posterior wall of the ventricle. This attachment is generally displaced toward the base of the heart with an excess of tension on the anterior leaflet and extreme limitation of posterior leaflet motion. The valve is most often predominantly regurgitant.
Regurgitant Mitral Valves with Normal Anatomy Associated with Congenital Cardiac Lesions
Isolated Annular Dilation; Isolated Elongation of the Chordae and/or the Papillary Muscle
There is no evidence of the congenital origin of these lesions. They are not found at birth unlike the previous anomalies described above. They are usually associated with significant volume loading of the left ventricle, that is large ventricular septal defect or large patent ductus arteriosus. Sometimes minor anomalies of the valvular tissue or the papillary muscles can give an indication toward a true congenital origin. The papillary muscle may have an ischemic aspect, and even rupture, this is mostly seen in neonates.
ALCAPA
The mitral regurgitation in patients with anomalous coronary artery from the pulmonary artery is of ischemic origin. The anatomy is normal. The functional classification is of systolic restriction of one of the segments of the posterior leaflet (Carpentier type IIIb).
Supravalvar Mitral Ring
Quoted as a common cause of congenital mitral valve stenosis, the supravalvar mitral ring is an acquired fibrous construction attached to the posterior annulus of the mitral valve and from both commissures to the mid-height of the anterior leaflet. The supravalvar mitral ring is secondary to turbulent flow through the mitral orifice. The primary lesion of the mitral valve responsible for the turbulent flow can be obvious, stenotic, or regurgitant or can be very discrete and difficult to identify. The supravalvar mitral ring is prone to reoccur after surgical resection, unless the underlying anatomical anomaly has been identified and corrected. The supravalvular ring can be encountered very early in life. It has to be suspected every time the transvalvular gradient increases during follow-up or when the Doppler gradient is greater than what the anatomy depicted with the echocardiographic study would suggest; sometimes, it is only found at operation.
Mitral Valve Disease with Excess Leaflet Tissue
They are Marfan syndrome, Loyes-Dietz syndrome, mitral valve prolapse, Barlow disease, Elher-Danlos syndrome, and mucopolysaccharidosis type I. All include elastic fibers alteration and myxomatous tissue proliferation of various degrees. Most are now well associated with chromosomal mutations.
Acquired Mitral Valve Disease
Rheumatic Heart Disease
Acute rheumatic fever (ARF) is an autoimmune disorder. The immune response to group A streptococcal M protein generates T cells and antibodies that cross-react with cardiac antigens. In some patients, the acute damage to the valves will induce chronic and evolving lesions secondary to the scarring process and/or the hemodynamic modifications. This is known as rheumatic heart disease.
Acute Lesions
Acute lesions are exclusively regurgitant. On inspection, the valvar tissue and the chordae are swollen but supple. Prolapse predominantly affects the anterior leaflet. This prolapse is usually related to large elongation of the marginal chordae that appeared stretched; chordal ruptures are rare. Multiple small nodules (2 to 3 mm diameter) can be seen on the free edge of either mitral leaflet. The annular dilation is secondary to the myocarditis.
Chronic Lesions
The healing of the spongiosa induces fusion of chordae as demonstrated by reduction in their number and increase of their thickness. The physiology of the regurgitation is always a combination of prolapse of the anterior leaflet, retraction of the posterior leaflet, and annular dilation. In the pediatric age group, the mitral valve is exclusively or predominantly regurgitant. The stenosis appears later and the age of apparition of the mitral stenosis varies greatly with the geographical origin of the population affected, suggesting different pattern of infection (i.e., age of first ARF episode) and influence of other factors (genetic mostly and alimentation).
Infective Endocarditis
Bacterial endocarditis of the mitral valve is rare. It is always a regurgitant lesion. At the Royal Children’s Hospital, Melbourne, in the last decade most patients had normal native mitral valve. It is very important for the surgeon to be able to differentiate intact valvar tissue, supple thin and resistant from infected tissue, thickened edematous and friable.
The Left Atrioventricular Valve in Repaired Atrioventricular Septal Defect
Anatomy
The anatomy of the left atrioventricular valve after complete AVSD repair has standard features: The superior and inferior bridging leaflets are partitioned transversally at the level of the crest of the septum. Their mobility is very limited at that level; it increases further away from the partition point. The superior and inferior bridging leaflets face one another through the zone of apposition made of by rough surface. The quality of the suspension apparatus to the free edge varies greatly from patient to patient. The zone of apposition between these two leaflets has often been closed with continuous or interrupted sutures at the time of the primary repair. In the most common configuration, the left lateral leaflet (LLL) or posterior leaflet is normally developed and the superior and inferior bridging leaflets separate from one another and delineate a triangular orifice at the inferior aspect of the left AV valve annulus. This orifice is covered with the LLL. It is triangular; the base hinges at the posterior aspect of the annulus and the tip faces exactly where the superior and inferior leaflets separate. Between one-third and one-fifth of the circumference of the reconstructed left AV valve belongs to the LLL. Two papillary muscles make the suspension apparatus. The anterior papillary muscle underneath the zone of apposition between the superior bridging leaflet and the LLL and the posterior papillary muscle
underneath the zone of apposition between the inferior bridging leaflet and the LLL and their respective commissure. The leaflet has a broad base when it covers one-third of the annulus or a narrow base when it covers one-fifth. In that configuration, the LLL is tall and narrow and unstable.
underneath the zone of apposition between the inferior bridging leaflet and the LLL and their respective commissure. The leaflet has a broad base when it covers one-third of the annulus or a narrow base when it covers one-fifth. In that configuration, the LLL is tall and narrow and unstable.
In the least common configuration (6% to 10% of the complete AVSD), the LLL is diminutive or absent. Both superior and inferior bridging leaflets are supported by the anterior papillary muscle, while the posterior papillary muscle is commonly diminutive or more rarely absent. Both bridging leaflets reach the left lateral annulus where a true commissure can be seen. Most often, the diminutive posterior papillary muscle is supporting a second orifice in the inferior bridging leaflet.
Mechanism of the Regurgitation in Repaired Left Atrioventricular Valve
Valves with Normally Developed Left Lateral Leaflet
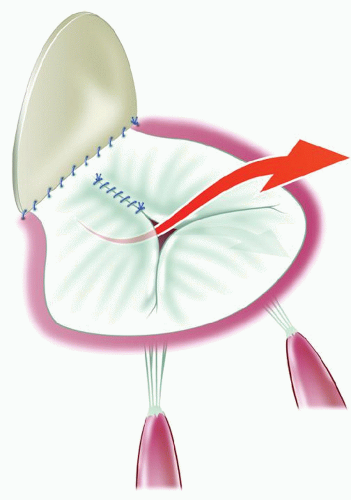
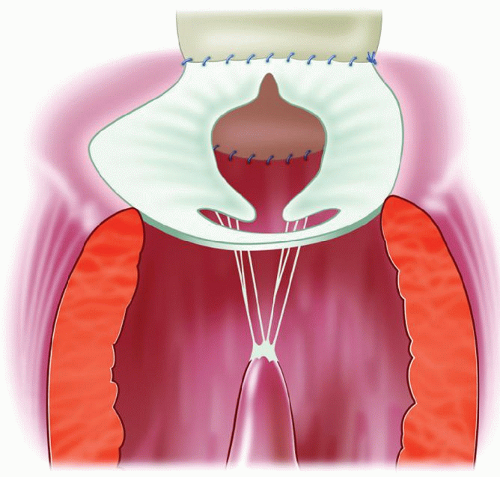
The regurgitation is related to the lack of apposition facing the tip of the LLL. The closure of the cleft does not restore a surface of apposition; in fact, the little apposition surface may be reduced and distorted by the cleft closure (Fig. 102.1). If the cleft closure has ruptured or partially ruptured, then the defect created and the doming of the combined bridging leaflets augments the regurgitation. If the regurgitation has been long-standing, the secondary lesion or dysplastic lesions on the edges of the cleft are severe with thickening, sometimes calcification and severe retraction of the leaflet tissue. However, it is almost the rule for the LLL to be thin and pliable with no secondary or dysplastic lesion. There is no restriction of leaflet motion and no prolapse.
Absent or Very Hypoplastic Left Lateral Leaflet
At the time of initial repair, the cleft closure is not performed or only partially executed in order to avoid the creation of a stenosis. Consequently, the regurgitation is always through the cleft, with retraction of the edges of the cleft and very few or no chordae attached to them (Fig. 102.2).
INDICATION AND PLANNING OF THE REPAIR
Echocardiography
The long axis view obtained from the apex or from subcostal view of the transthoracic study is best to grade the regurgitation, provide an accurate estimation of transvalvular gradient and define the precise amplitude of any prolapse or restriction. The short axis view gives a direct evaluation of the area of the mitral orifice, a precise localization of the regurgitant jet. It allows an analysis of the papillary muscles (presence, size, location, and symmetry). The transeosophagal echo is superior for the anatomical details of the suspension apparatus, the evaluation of the functional classification in relation to the anatomy (response to the question: how much prolapse/restriction and where?), but it is less useful to grade the severity of the regurgitation. The transgastric position allows for a short axis cut with precise measurement of the shortening fraction and an en face view of the mitral valve.
For mitral stenoses, the peak instantaneous and mean gradients across the valve have to be interpreted according to the quality of the diastolic function of the heart and the associated lesions (mainly Qp/Qs, the presence of an intra-atrial shunting and gradient across the foramen ovale/atrial septal defect). The overall impact of the gradient on the surgical indication has to be weighted with the pulmonary artery pressure but mostly the clinical tolerance.
Functional Classification
Transthoracic and transoesophageal echocardiography allows classifying the malformations according to the motion of the leaflets in one of the three following types:
Type I: Normal leaflet motion. The regurgitation results from a lack of coaptation between the leaflets.
Type II: Leaflet prolapse. The free edge of one or the two leaflets overrides the plane of the orifice during systole.
Type III: Restricted leaflet motion. The motion of one or the two leaflets is limited.
This can be secondary to short or stiff leaflet tissue or suspension apparatus (type IIIa) or the leaflet can be pulled away from the coaptation area by a paradoxical motion of the ventricular wall (type IIIb or systolic)
Other Investigations
Catheter study and angiography generate no additional information to the echocardiography and should not be performed.
Magnetic Resonance Imaging allows precise calculation of the ventricular volumes irrespective of the septal geometry; this may help for the decision-making with small left ventricle in mitral valve stenosis. The regurgitation fraction is measured accurately. Gradients and flows are demonstrated with MRI. In small patients, MRI does not help with the analysis of the valve anatomy.
Three-dimensional echocardiography is progressing rapidly with the exponential increase of computer power and miniaturization of the probes. The information generated are, however, of little use in small patients as the spatial resolution is still insufficient. Generally, the most obvious benefit is the ability to locate precisely the types II and III on the 3D en face view while they are quantified much more precisely on the 2D.
Indications
The indication for surgical intervention has to weigh several considerations.
According to the Mitral Valve Annulus
Large Mitral Valve Annulus (>30 mm in Female Patients and 32 mm in Males).
Using a wide range of mitral valve repair techniques, the probability of a successful repair of the valve is very high. A remodeling annuloplasty will not be outgrown and will not generate stenosis with the growth of the patient. The surgical indication is similar to the current indications in the adult population: The patients should be operated on as soon as the volume of the regurgitation is severe, irrespective of the severity of symptoms. The probability of repair is directly related to the experience of the surgical team but the repair of virtually all valves is an accessible goal.
Mitral Valve Annulus <18/20 mm
Biventricular repair should be considered only if the mitral valve annulus is not hypoplastic (Z value greater than −1.5). The repair is technically very challenging while the replacement is only possible with the use of surgical artifacts associated with significantly increased mortality. In these patients, the surgical indication should be differed as long as the patient can be managed with intense medical therapy, including transfusion. Aggressive medical therapy allows delaying the surgery for several months in some instances and can generate significantly more favorable operating conditions. However, the requirement for positive pressure ventilatory support more invasive than continuous nasal flow (either CPAP or other mode of pressure support) should trigger the surgical indication and so would a flat weight curve.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree