Fig. 13.1
Graphic depiction of the mid-systolic click followed by a high pitched murmur as seen with mitral valve prolapse. Since the murmur is due to regurgitation, the most effective location to listen for it is at the apex of the heart
The Valsalva maneuver altered the systolic click such that it came earlier and the murmur lasted longer, while the opposite occurred with forced expiration, as there was a late systolic click and a shorter murmur.
Test Results
The ECG was normal.
A CT scan (Fig. 13.2) as well as a transesophageal echocardiogram (Fig. 13.3) were performed. The CT showed a dissection of the aortic arch with blood entering the media forming a false lumen and the echocardiogram showed prolapse of a mitral valve leaflet into the left atrium.
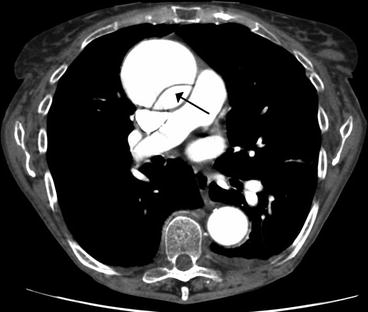
Fig. 13.2
CT of a Stanford type A aortic dissection of the aortic arch (Image courtesy of James Heilman, MD. Licensed under Creative Commons Attribution-Share Alike 3.0 via Wikimedia Commons – http://commons.wikimedia.org/wiki/File:DissectionCT.png#mediaviewer/File:DissectionCT.png)
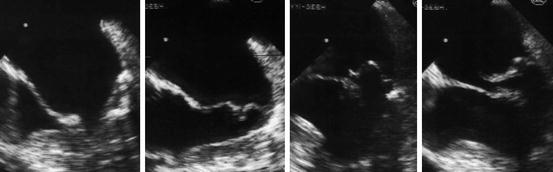
Fig. 13.3
Transesophageal echocardiogram of the mitral valve with >2 mm displacement of the leaflets beyond the annulus into the left atrium, indicating MVP (Image courtesy of J. Heuser. Licensed under Creative Commons Attribution-Share Alike 3.0 via Wikimedia Commons – http://commons.wikimedia.org/wiki/File:Mitralinsuff_TEE.jpg#mediaviewer/File:Mitralinsuff_TEE.jpg)
Clinical Basics
Normal Anatomy
The mitral valve is located at the transitional zone between the left atrium and ventricle, consisting of four anatomical structures: annulus, leaflets, chordae tendineae and papillary muscles.
The mitral valve annulus is a junctional ring that not only separates, but gives attachment to the left ventricle and left atrium, while serving as a fulcrum for the anterior and posterior mitral leaflets. The anterior border corresponds to the base of the anterior leaflet. The remainder of the border, composed of muscular tissue, is more dynamic and represents most of the annular circumference, corresponding to the attachment of the posterior leaflet. The annulus moves down toward the LV apex in systole and up toward the LA in diastole due to the contraction of the papillary muscles [3].
The anterior leaflet (Fig. 13.4), which has a rounded edge appearance, occupies approximately a third of the annular circumference, and the posterior leaflet, which occupies the remaining circumference, appears long and narrow. The anterior leaflet has greater mobility and the posterior leaflet plays more of a supportive role [3].
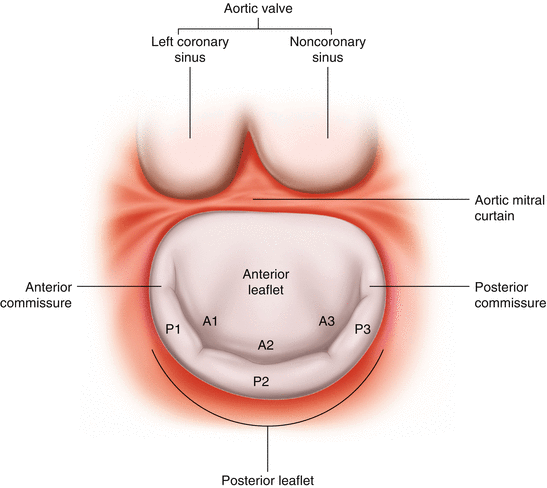
Fig. 13.4
Mitral valve: segmental anatomy. The Carpentier nomenclature categorizes each leaflet into three segments. The posterior leaflet is termed P and comprises P1, P2, and P3 corresponding to lateral, middle, and medial scallops. The anterior leaflet segments are termed A1, A2, and A3 and correspond to the anterior leaflet scallops
The chordae tendinae (Fig. 13.5) are subdivided into those attached to the free edge of the leaflet which are the majority, and those that are secondary, being attached to the rough zone of the leaflets (and having an anchoring role) [3]. The main role of the chordae is to prevent the free margins of the valve from protruding beyond the annulus into the left atrium during systole [4].
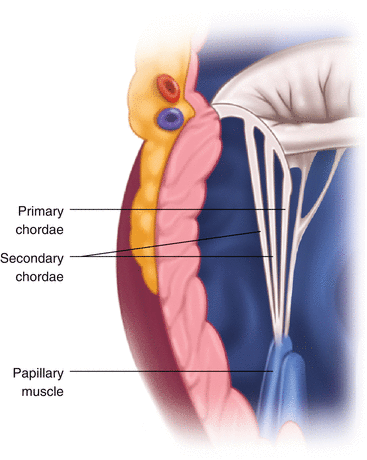
Fig. 13.5
Mitral valve: subvalvular apparatus. The papillary muscle gives rise to the primary chordae tendineae which attach to the medial region of the anterior and posterior leaflets and the secondary chordae are attached to the more lateral region of the leaflet
The two papillary muscles emerge as single bodies from the LV wall and then divide into a variable number of heads, serving as anchors for the chordae [3]. The ventricular contraction originates in the papillary muscles allowing for increased tension in the chordae to prevent significant amounts of mitral regurgitation (MR) of blood back into the left atria. The posterior papillary muscle is supplied by the right coronary artery in most people or from the third obtuse marginal of the left circumflex artery. The anterior papillary muscle is located on the anterolateral LV wall and has an anastomosing blood supply from the obtuse marginal branch of the circumflex coronary artery and the first diagonal branch from the left anterior descending artery [3]. Thus, after a myocardial infarction the posterior papillary is much more likely to be damaged and result in MVP since there is not anastomosis of blood supply.
Patients with MVP typically have a normal ECG, but sometimes there is ST and T wave depression or T-wave inversion in the inferior leads. This is thought to reflect ischemia created when the prolapsed valve exerts excessive tension on the papillary muscles which stretches the myocardium [1].
Etiology
MVP is the most common cause of significant MR and if papillary muscle rupture does occur, severe regurgitation will result and can lead to cardiogenic shock.
Individuals with Ehler-Danlos Syndrome, Marfan syndrome or polycystic kidney diseases are at a higher risk of developing MVP during their lives.
Signs and Symptoms
The most common complaints of patients with MVP include heart palpitations, dyspnea, fatigue and sometimes chest pain that may be present on physical exertion that is sharp, non-radiating and prolonged [1].
Prevalence
MVP has a prevalence in the general population of 2.4 %, of which 1.3 % are classical and 1.1 % are non-classical prolapse [5].
Key Auscultation Features
During systole, one or both of the leaflets of the mitral valve will protrude beyond the annulus into the left atrium. Crisp nonejection clicks are heard and the systolic murmur will endure until the A2 component of the second heart sound. Most commonly, a redundant posterior leaflet will balloon into the left atrium. There are variations in the auscultation findings noted below: [2].
Midsystolic nonejection click without a late systolic murmur.
Midsystolic nonejection click with a late systolic murmur.
Late systolic murmur alone.
The sound that precedes the systolic murmur is called a nonejection click or sound. It should not be called mid-systolic because the click can occur as early as ejection and as late as a widely split S2. It is not uncommon for there to be multiple clicks. If the etiology of the mitral valve prolapse is a myocardial infarction with or without papillary muscle damage, it is unlikely that there will be a click with the murmur [2].
There are a few theories on the origin of the nonejection click that precedes the systolic murmur [2]:
Chordal Snap Theory: At the point of peak pressure in midsystole, there is a click caused by the sudden stretch of chordae. This theory has been challenged because the papillary muscles contract early putting the chordae under too much pressure to contribute to the click sound.
Valvular: Due to redundant valve tissue or an abnormality of chordal length there is a valvular sound produced when there is a loss of support of one leaflet by its opposing leaflet. The onset of the mitral valve prolapse murmur is proceeded by the click even though the reverse appears to be the case on auscultation.
Contraction Ring Theory: There is excessive contraction during midsystole of the left ventricle in a posteroinferior area. This pushes the posterior papillary muscle allowing the chordae to lose tension. With continued systole the chordae then tighten once again, producing the click. This is supported by angiogram evidence showing that 80 % of patients with mitral valve prolapse have LV wall abnormality.
The late systolic isolated MR murmur may be associated with significant adverse LV remodeling, and should be considered evidence of hemodynamically important MR [6].
Late systolic murmurs are described as soft or moderately loud, high-pitched murmurs at the left ventricular apex that start well after ejection and end before or at the second heart sound. They can occur with or without mid-systolic clicks, and result from late-systolic regurgitation due to mitral valve leaflet prolapse [6].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


