Mitral Valve Disease
Leonardo Rodriguez
A. Marc Gillinov
With the widespread availability of echocardiography, there has been an increase in the number of patients diagnosed with mitral valve dysfunction. Although the prevalence of mitral stenosis caused by rheumatic disease has decreased in recent years in the United States and Europe, the prevalence of mitral regurgitation (MR) has increased. In adults, the most common causes of MR are degenerative, ischemic, endocarditic, and rheumatic processes (1). The etiology of mitral valve dysfunction influences the therapeutic approach, timing of intervention, results of treatment intervention, and long-term survival. Therefore, after a brief review of mitral valve anatomy, we discuss the management of mitral valve disease, focusing on the etiologies of mitral valve dysfunction.
Mitral Valve Anatomy
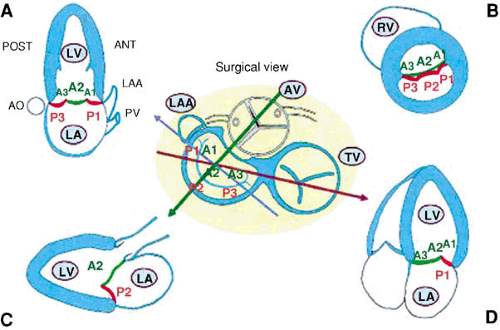
The mitral apparatus includes the leaflets, annulus, chordae tendineae, papillary muscles, and left ventricle.
Leaflets
The mitral valve has two leaflets, the anterior (aortic) and posterior (mural). The leaflets are attached to the mitral annulus and the to the papillary muscles by primary and secondary chordae. The anterior mitral leaflet is in direct continuity with the fibrous skeleton of the heart.
The posterior leaflet is rectangular. The free margin of the posterior leaflet has two clefts that divide the posterior leaflet into three scallops: the largest or middle scallop, the posteromedial scallop, and the anterolateral scallop. Motion of the posterior leaflet is more restricted than that of the anterior leaflet; however, both mitral leaflets contribute to effective valve closure.
Annulus
The mitral annulus is the site of leaflet attachment to muscular fibers of the atrium and ventricle. The annulus is a nonplanar, saddle-shaped structure (2). It is flexible and decreases in diameter during systole by approximately 26% (3,4). Anteriorly, the annulus is attached to the fibrous skeleton of the heart (2). This limits flexibility and the capacity of the anterior annulus to dilate with MR, although dilatation of the anterior annulus has recently been documented in patients with MR (5,6). Important anatomic relations of the mitral annulus include the circumflex coronary artery, the coronary sinus, the aortic valve, and the bundle of His.
Chordae Tendineae
The chordae tendineae are chords of fibrous connective tissue that attach the mitral leaflets to either the papillary muscles
or the left ventricular (LV) free wall. The chordae are divided into primary, secondary, and tertiary chordae. Primary chordae attach to the fibrous band running along the free edge of the leaflets, ensuring that the contact surfaces of the leaflets coapt without leaflet prolapse or flail. Secondary chordae attach to the ventricular surface of the leaflets and contribute to ventricular function (7,8). Tertiary chordae are unique to the posterior leaflet. They arise directly from the LV wall or from small trabeculae to insert into the ventricular surface of the leaflet near the annulus.
or the left ventricular (LV) free wall. The chordae are divided into primary, secondary, and tertiary chordae. Primary chordae attach to the fibrous band running along the free edge of the leaflets, ensuring that the contact surfaces of the leaflets coapt without leaflet prolapse or flail. Secondary chordae attach to the ventricular surface of the leaflets and contribute to ventricular function (7,8). Tertiary chordae are unique to the posterior leaflet. They arise directly from the LV wall or from small trabeculae to insert into the ventricular surface of the leaflet near the annulus.
Papillary Muscles
The anterolateral and posteromedial papillary muscles each supply chordae tendineae to both leaflets. The anterolateral papillary muscle receives a dual blood supply from the anterior descending coronary artery and either a diagonal branch or a marginal branch of the left circumflex artery (9,10). The posteromedial papillary muscle receives its blood supply from either the left circumflex artery or a distal branch of the right coronary artery. Because of its single blood supply, the posteromedial papillary muscle is more commonly affected by myocardial infarction (MI).
Acute and Chronic Mitral Regurgitation
Acute Mitral Regurgitation
Pathophysiology
The pathophysiologic consequences of acute, severe MR—such as that observed in patients following rupture of a papillary muscle—differ from those of chronic MR. With acute MR, a sudden volume overload is imposed on the nondilated, nonhypertrophied left ventricle and left atrium causing a sudden increase in left atrial pressure. Preload is increased, whereas afterload decreases as a result of the newly developed low-pressure runoff to the left atrium during systole (11). In the absence of coronary artery disease or MI, LV contractile function can be normal or even supranormal secondary to augmented sympathetic nervous stimulation of the myocardium (11,12).
Despite increased LV preload and contractility and decreased afterload, overall LV pump function declines in acute MR. Total LV stroke volume increases, but much of this flow is directed into the left atrium diminishing the effective, forward stroke volume through the aorta. The clinical impact of acute MR is largely determined by the compliance of the left atrium. In a normal, relatively noncompliant left atrium, acute MR results in high left atrial pressure, which can rapidly lead to pulmonary edema.
Diagnosis
Acute MR is usually associated with the sudden development of severe LV failure. Patients are often in pulmonary edema when initially seen, and cardiogenic shock is common. Severe acute MR secondary to acute inferoposterior MI is seen more often in women (13).
The physical examination is dominated by findings associated with LV failure; tachycardia and tachypnea are common. The murmur is usually loud if normal LV function is present. The murmur may be soft if LV function is markedly reduced.
Echocardiographic studies demonstrate the etiology of the acute regurgitant lesion: vegetations in patients with infectious endocarditis, ruptured chordae in individuals with mitral valve prolapse, or papillary muscle rupture in AMI. In many patients in critical condition, transthoracic echocardiography may show the presence of MR, but tachycardia or poor windows in a ventilated patient may obscure the precise structural mechanism. Transesophageal echocardiographic examination reveals the cause of the acute MR in almost all cases. Transesophageal echocardiography also allows evaluation of left and right ventricular function and other valvular lesions.
Bedside right heart catheterization is useful in these patients, allowing measurement of right-sided and capillary wedge pressures, as well as cardiac index. Patients with acute MR have marked elevation of filling pressures and low cardiac index. Large, regurgitant CV waves are observed in the pulmonary capillary wedge pressure tracing. Catheterization is also helpful in monitoring response to aggressive therapy.
Treatment
In patients with acute MR, IV vasodilator therapy with nitroprusside can produce dramatic benefit with reductions in ventricular cavity dilatation, diastolic filling pressures, and mitral regurgitant orifice (14,15). The nitroprusside infusion is continued until the patient is stabilized by either surgical intervention (14,16) or long-term oral medical therapy (e.g., angiotensin-converting enzyme inhibitors, digoxin, and diuretics). IV nitroglycerin is often effective in reducing pulmonary vascular congestion in these patients, particularly if the underlying etiology for MR is ischemic heart disease. Surgical therapy is almost always required in patients with acute severe MR. Intraaortic balloon counterpulsation can be helpful in stabilizing the patient before definitive therapy is instituted (13).
The surgical approach and results depend on the underlying pathology. Patients with MR secondary to AMI have a high surgical mortality (13). Patients with acute endocarditis may have elevated morbidity, particularly if there is multivalvular involvement, septic shock, or multiple septic emboli.
Chronic Mitral Regurgitation
The vast majority of patients present with chronic MR at the time of diagnosis with LV and left atrial adaptation to volume overload. The causes of MR are listed in Table 22.1.
Pathophysiology
MR causes LV and left atrial volume overload. The degree of volume overload depends on the regurgitant volume. The regurgitant volume is determined by the size of the regurgitant orifice area (ROA), the duration of systole and the systolic pressure gradient between the left ventricle and left atrium:
where MRV is the regurgitant volume, MROA is the regurgitant orifice area, C is a constant, TS is the duration of systole, LVP is the mean LV pressure, and LAP is mean LA pressure (17).
Initially, as the severity of MR increases there is progressive enlargement of the left atrium and left ventricle to compensate for the regurgitant volume. LV dilatation occurs as a result of remodeling of the extracellular matrix with rearrangement of myocardial fibers, in association with the addition of new sarcomeres in series and the development of eccentric LV hypertrophy (17). In patients with severe, chronic MR, the left atrium is markedly dilated and atrial compliance is increased. Although there is fibrosis of the atrial wall, left atrial pressure can be normal or only slightly elevated.
The ejection fraction may be normal or even hyperdynamic as the ventricle empties into the lower pressure left atrium. The increase in end-diastolic volume and the small end systolic volume help to maintain a normal forward stroke volume.
In the absence of primary myocardial damage, diastolic function of the dilated ventricle is normal with decreased chamber stiffness but normal myocardial stiffness. In the compensated phase, total stroke volume is enhanced and ventricular afterload normalizes. Over time, and for poorly understood reasons, the ventricle progressively dilates, has increased diastolic pressure and afterload, and reduced ejection fraction (17). During this decompensated phase, there is also significant left atrial enlargement, increase in capillary wedge pressure, and development of pulmonary hypertension and right ventricular dysfunction.
In the absence of primary myocardial damage, diastolic function of the dilated ventricle is normal with decreased chamber stiffness but normal myocardial stiffness. In the compensated phase, total stroke volume is enhanced and ventricular afterload normalizes. Over time, and for poorly understood reasons, the ventricle progressively dilates, has increased diastolic pressure and afterload, and reduced ejection fraction (17). During this decompensated phase, there is also significant left atrial enlargement, increase in capillary wedge pressure, and development of pulmonary hypertension and right ventricular dysfunction.
TABLE 22.1 Causes of MR | ||
|---|---|---|
|
The assessment of contractility in the presence of severe MR remains problematic; ejection phase indices overestimate contractile state (18). Ejection fraction is the most common parameter used to assess LV function but does not detect early changes in LV function. A recognized drop in LV ejection fraction is a late event. Several complex methods such as end systolic stress and elastance have been used to assess LV function but are impractical for routine evaluation and serial follow up of patients (19).
Diagnosis
Echocardiography is the main diagnostic tool in patients with MR because it displays in real time valvular anatomy and function and provides information about left and right ventricular function. The left ventricle can be normal in size or dilated depending on the severity and duration of MR. The left atrium is also enlarged, more severely in the presence of atrial fibrillation.
A systematic and comprehensive approach using transthoracic echo is of critical importance (20) (Fig. 22.1). The complete mitral valve apparatus must be interrogated by two-dimensional echo and Doppler. Assessment of global and regional LV function is important to understand the mechanism and prognosis of MR. New techniques such as tissue Doppler or strain have been applied in asymptomatic patients to assess subclinical LV dysfunction. The initial results are encouraging (21,22). In experienced echo laboratories transthoracic echocardiography may be adequate for diagnostic evaluation in the majority of patients with MR. Transesophageal echocardiography offers a better definition of the anatomy, and is indicated in cases with suboptimal transthoracic windows or unclear etiology, mechanism, or severity of the MR.
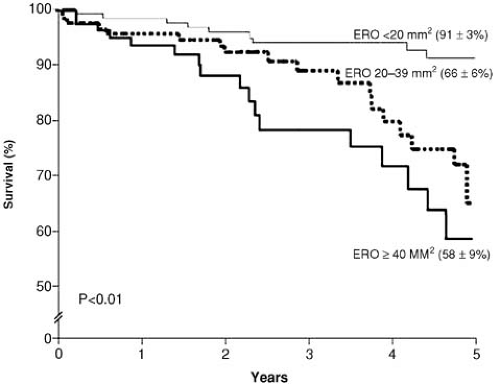
The severity of MR can be assessed by semiquantitative methods based on the size (length, width, or area) of the regurgitant jet or the width of the vena contracta or, alternatively, by quantitative techniques that attempt to quantify the regurgitant volume and fraction and the size of the ROA (23). The importance of quantification has been reinforced by recent data suggesting that large ROAs are associated with poor prognosis even in asymptomatic patients (24) (Fig. 22.2). Patients with ischemic MR have a risk ratio of cardiac death of 2.38 if the regurgitant orifice is larger than 20 mm2 (25,26). Comparatively, patients with degenerative MR and ROA greater than 40 mm2 carry an unfavorable prognosis. This difference can be partially explained by reduced ejection fraction in the ischemic group (24,27).
A consensus on quantification of native valvular regurgitation using echocardiography has been published (23) (Table 22.2). A regurgitant volume greater than or equal to 60 mL and an effective ROA greater than or equal to 40 mm2 characterize severe MR. In most patients, a comprehensive evaluation using multiple techniques and parameters is necessary for accurate determination of the severity of MR. Very eccentric jets of MR are always a challenge for quantification and require considerable expertise.
Stress echocardiography can be used to determine the functional significance of MR, particularly in patients with minimal or no symptoms. It is also helpful to uncover latent LV dysfunction (28). Exercise ejection fraction predicts postoperative LV function better than does the resting ejection fraction (28). An increased end systolic volume with exercise is also a predictor of diminished post operative ejection fraction (28). In patients with tricuspid regurgitation, it is also possible to assess the development of pulmonary hypertension with exercise.
Treatment
All patients with MR should receive antibiotic prophylaxis before dental and other invasive procedures according to American Heart Association/American College of Cardiology guidelines (29). In addition, without antibiotic prophylaxis patients with mitral valve prolapse have a three- to eightfold (29) higher risk of developing infective endocarditis (30).
Optimal blood pressure control is important. Hypertension is a risk factor for development of severe MR in patients with mitral prolapse (31) and negatively affects LV performance in patients with ischemic heart disease or dilated cardiomyopathy.
Restriction of physical activity is recommended only in symptomatic patients and in those with LV dysfunction. Isometric exercise should be discouraged. The 2005 task force 3 issued recommendation for athletes with MR. Athletes with severe MR and definite LV enlargement (≥60 mm), pulmonary hypertension, or any degree of LV systolic dysfunction at rest should not participate in competitive sports (32).
Management of underlying disease is fundamental in patients with ischemic heart disease or infective endocarditis. Other less frequent causes of secondary MR are affected by progression of the underlying process such as lupus or other inflammatory processes.
The most important goals of surgical intervention are improvement of symptoms, preservation of LV function, and increased longevity. Other considerations include preservation of the native mitral valve apparatus, avoidance of chronic anticoagulation, maintenance and/or restoration of sinus rhythm, and prevention/improvement of pulmonary hypertension and right ventricular dysfunction.
Degenerative Mitral Valve Disease
Degenerative mitral valve disease includes myxomatous mitral disease, mitral leaflet flail or prolapse, and Barlow syndrome. The Framingham Heart Study employed strict echocardiographic criteria to determine the prevalence of mitral valve prolapse. They noted a prevalence of 2.4% in their population; 60% of the patients with mitral valve prolapse were women (33).
TABLE 22.2 Qualitative and Quantitative Parameters for Grading Mitral Severity | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Etiology and Pathology
Pathologic changes associated with degenerative mitral valve disease may include annular dilatation, leaflet thickening, myxoid degeneration, chordal elongation, chordal rupture, and annular and leaflet calcification (34). In patients with degenerative disease, the mitral leaflets and chordae have 3% to 9% higher water contents and 30% to 150% higher concentrations of glycosaminoglycans than do normals (35). There are lower collagen concentrations in the leaflets compared with normals. The biochemical effects are more pronounced in chordae than in leaflets.
Disruption of the collagen bundles may explain why degenerative leaflets and chordae exhibit enhanced extensibility and decreased stiffness compared to normal valves. Chordal rupture is one of the causes of severe MR in degenerative disease and is secondary to mechanical weakening of the chordae and the abnormal stresses imparted by the redundant leaflet (36). Flail leaflet is more common in patients with unileaflet prolapse (37).
Mitral valve prolapse has been associated with a number of different conditions, including Marfan syndrome, Ehlers-Danlos syndrome, acute rheumatic carditis, and a variety of congenital cardiac anomalies. A number of these entities are the result of genetic defects in connective tissue structure that produce, among other anatomic abnormalities, mitral valve prolapse. Although most cases of degenerative mitral valve disease are sporadic, a familial basis for the condition has been recognized. This form of mitral valve prolapse has an autosomal dominant mode of inheritance with variable penetrance and is influenced by age and gender. There is marked heterogeneity of clinical presentation (36). A locus for autosomal dominant myxomatous mitral valve prolapse, MMVP1, has been mapped to chromosome 16p11.2-p12.1 (38) and a second locus, MMVP2, to chromosome 11p15.4 (39).
Natural History
The clinical spectrum of patients with degenerative valve disease is wide. Two distinct clinical groups appear to exist. One consists of young women with a midsystolic click and mild echocardiographic prolapse; they often have multiple complaints. The other group consists of middle-aged men with thickened valves, more severe prolapse, and MR.
The majority of patients with mitral valve prolapse and no or mild MR are asymptomatic (40,41,42). Many patients have symptoms that seem unrelated to the cardiac pathophysiology. For example, anxiety, easy fatigue, palpitations, and orthostatic hypotension have all been linked to this syndrome. Some authorities ascribe these symptoms to dysfunction of the autonomic nervous system with inappropriately increased sympathetic nervous activity at rest and with mild exertion (43). The exact relationship of these autonomic abnormalities to mitral valve prolapse is unclear. Studies that included asymptomatic patients with mitral valve prolapse have found no evidence of abnormal autonomic or neuroendocrine function either at rest or during tilt testing.
Some patients report chest discomfort that, at times, is obviously musculoskeletal in origin and at other times seems anginal in nature. It has been suggested that anginal chest discomfort results from abnormal tension and traction on papillary muscles (44).
The majority of patients with mitral valve prolapse and no or mild MR have a benign prognosis, but there are a number of complications that have been associated with this entity
including arrhythmias, sudden death, cerebrovascular events, and MR.
including arrhythmias, sudden death, cerebrovascular events, and MR.
Arrhythmias are common in patients with mitral valve prolapse. Both supraventricular and ventricular arrhythmias have been reported; however, it is unclear to what degree mitral valve prolapse is the actual cause of these rhythm disturbances.
Mitral valve prolapse carries a small but real risk of sudden death. The presence of severe MR with ruptured chordae and a flail segment increases the risk of sudden death. In Mayo Clinic data based on 348 patients with a follow-up of 48 months, the rate of sudden death was 1.8% per year (45). By multivariate analysis, independent predictors were functional class, ejection fraction, and atrial fibrillation. In patients with no or minimal symptoms, sinus rhythm and normal LV function, a linearized rate of 0.8% per year was observed; in patients in functional class III or IV, the incidence of sudden death was 7.8% (45).
Cerebral embolism occasionally occurs in patients with mitral valve prolapse. Formation of platelet–fibrin thrombi on severely myxomatous valves has been proposed as the embolic source. AMI can be another manifestation of arterial embolism in these patients. Zuppiroli et al. (40) followed 300 patients with mitral valve prolapse for more than 8 years and found a serious complication rate of only 1% per year in this population. The increased risk of cerebrovascular events in patients with mitral valve prolapse appears to be limited to older patients with thickened valves and MR (46,47,48,49).
Increasingly severe MR occurs in 10% to 15% of patients and can require mitral valve surgery if symptoms or LV dysfunction develop (40,42). Severe MR requiring surgery is three times more common in men than in women. Correlates with severe MR and the need for mitral valve surgery include male gender, older age, and the presence of obesity and hypertension (31).
Diagnosis
Physical Examination
Patients with mitral valve prolapse are often asthenic in habitus with low body weights. Arterial blood pressure is frequently low and orthostatic hypotension may be present. Straight-back syndrome, pectus excavatum, scoliosis, and a narrow thoracic anteroposterior diameter may be present. If severe MR is present, the carotid pulse may be brisk in upstroke with a hint of rapid fall off. Palpation of the precordium may reveal a brief inward movement of the apical impulse in midsystole coinciding with the occurrence of the midsystolic click.
Auscultation of these patients is best performed using the diaphragm of the stethoscope with the patient lying and standing. The usual finding is a sharp, systolic click heard 0.14 seconds or more after the S1. This click differs from the ejection click of aortic valve disease because it occurs well after the onset of the carotid arterial upstroke. At times, more than one click is heard. It is thought that clicks are generated by tensing of the chordae tendineae and billowing of the mitral valve leaflets. The click(s) is usually, but not always, followed by a mid- to late systolic crescendo murmur heard best at the apex. The duration of the murmur correlates directly with the severity of MR; the earlier and more prolonged the murmur, the more severe the regurgitation. The auscultatory findings are dynamic and change depending on loading conditions. Thus, maneuvers or conditions that modify LV size affect the timing and duration of the murmur. A decrease in cavity size moves the click and murmur earlier in systole because the valvular prolapse occurs earlier. Smaller ventricular size occurs with dehydration, standing, amyl nitrate administration, and the Valsalva maneuver. Although the murmur is longer in duration, its intensity may be less because the severity of MR diminishes in these conditions. Maneuvers that increase LV cavity size (increases in systemic blood volume, decreases in myocardial contractility, and increases in venous return) move the click and murmur later in systole.
Echocardiography
Echocardiography is the primary diagnostic modality in patients with degenerative mitral valve disease and confirms the diagnosis of mitral valve prolapse by demonstrating more than a 2-mm systolic posterior displacement of one or both leaflets into the left atrium in the parasternal long axis view (50,51). The echocardiogram determines the mechanism(s) and severity of MR and enables assessment of other valves and measurement of LV function and dimensions. In patients with classic prolapse, the leaflets are thick and redundant with variable degrees of billowing into the left atrium. Patients with mitral valve prolapse but without leaflet thickening have a more benign prognosis (52).
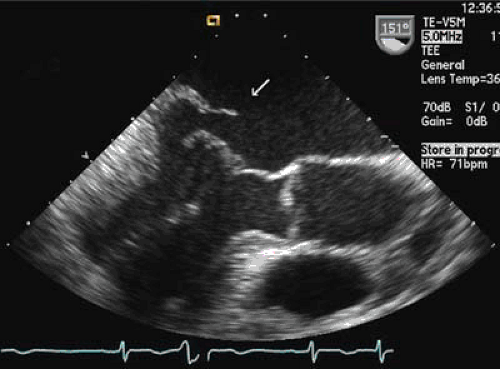
The ejection fraction is usually high and an ejection fraction below 60% should be considered abnormal in patients with severe MR. In cases of severe mitral valve prolapse, it is possible to observe a coaptation gap during systole. In many patients it is also possible to visualize ruptured chordae and flail segments using transthoracic echocardiography. A systematic approach using standard transthoracic echocardiography allows determination of the mechanism and severity of MR in most cases (20) (see Fig. 22.1). Transesophageal echocardiography is helpful in cases of suboptimal windows and, for better definition of the size and extent of the flail segment (Fig. 22.3). Intraoperative transesophageal echocardiography is an essential tool in patients undergoing mitral valve repair.
Color Doppler echocardiography allows visualization of the origin, direction, and severity of the regurgitant jet. In most cases the jet has a central origin with involvement of either the central scallop of the posterior leaflet (P2) or the central portion of the anterior leaflet (A2). The direction of the jet is typically opposite to the leaflet involved. In cases of predominant posterior leaflet involvement, the jet is anteriorly directed, and the jet is posteriorly directed when the prolapse is anterior. In cases of balanced bileaflet prolapse the jet is central. Involvement of commissural scallops may direct the jet in any direction.
When surgical therapy is planned, knowledge of the coronary artery anatomy is essential. In patients at low risk for coronary artery disease, this may be achieved with multislice CT scanning. In patients at high risk for coronary artery disease, coronary angiography is indicated (53,54).
Medical Treatment
As mentioned, patients with MR or thickened leaflets should receive antibiotic prophylaxis. Patients with mitral valve prolapse but without regurgitation have an excellent prognosis. They usually do not progress to severe MR. Periodic echocardiographic follow-up in these patients is prudent.
The routine use of vasodilators in patients with MR remains controversial. Although there is agreement that this therapy is of value in patient with LV dysfunction (55,56,57), its indication in asymptomatic patients with normal ventricular function is less clear (57). In dogs with myxomatous disease, enalapril does not delay the onset of congestive heart failure (58). There is concern that vasodilators could mask the development of LV dysfunction and delay mitral valve surgery. Therefore, in the absence of adequate randomized clinical trials, the use of vasodilator therapy in asymptomatic patients with severe MR and normal LV function should be determined on a case by case basis taking into account LV size and systemic blood pressure. In patients with LV dysfunction at presentation therapy with vasodilators should be started and surgical correction performed without delay.
Surgical Treatment
Indications
Surgery should be considered in all patients with severe MR caused by degenerative mitral valve disease. More liberal indications for surgery are the result of increased understanding of the unfavorable natural history of patients with severe MR, extremely low operative risk in most patients with degenerative mitral valve disease, and excellent long-term results obtained with mitral valve repair (1,34,59). Definite indications for surgery in patients with severe MR are presence of symptoms or LV dysfunction (ejection fraction <0.60, end-systolic dimension >40–45 mm). Surgery should also be offered to patients with severe MR and new-onset atrial fibrillation.
There is some controversy concerning the role of surgery in asymptomatic patients with normal LV function. Recent data demonstrate high repair rates (>90%) and virtually no operative mortality in such patients (34,59,60). Furthermore, natural history studies demonstrate that asymptomatic patients with severe MR (effective ROA ≥ 40 mm2) have reduced survival; surgical repair in such patients is associated with improved survival (24). In this group of patients, the 5-year probability of death or late cardiac surgery was 84% (24). Such patients frequently have a flail leaflet, which is a marker of severe MR (61). These data support the use of quantitative echocardiography to assess the severity of MR in asymptomatic patients and early surgery in those with severe MR.
In summary, the decision for surgical repair in patients with degenerative MR should be based on symptoms, LV function (at rest and exercise), severity of MR and local operative mortality and rate of successful repair.
Approach
Mitral valve repair is preferred to mitral valve replacement in patients with degenerative mitral valve disease (60,62,63). The probability of valve repair depends on the experience of the surgical team and the pathology encountered. Segmental posterior leaflet prolapse is the most common finding, affecting 60% to 80% of patients with severe MR caused by degenerative disease (1,59,64). This is usually repaired by quadrangular resection and annuloplasty. In the setting of excess leaflet tissue and a tall posterior leaflet, this repair technique may result in LV outflow tract obstruction caused by systolic anterior motion (SAM) of the mitral valve. SAM is avoided by reducing the height of the posterior leaflet with a sliding repair, which moves the point of leaflet coaptation posteriorly, away from the LV outflow tract (65,66). Anterior leaflet repair is more challenging. Anterior leaflet pathology may be corrected with a variety of repair techniques including creation of artificial chordae, chordal transfer, the edge-to-edge repair, chordal shortening, and anterior leaflet resection (67,68,69,70,71,72). All mitral valve repairs include annuloplasty. Functions of the annuloplasty include improved leaflet coaptation by reduction of the septal-lateral dimension of the valve, reduced tension on suture lines, and prevention of future annular dilatation (73,74,75). A variety of annuloplasty techniques are available (partial versus complete; flexible versus rigid), and all appear to function well in patients with degenerative disease (64). However, failure to incorporate an annuloplasty in the repair jeopardizes durability (34). In rare instances, mitral valve repair is not feasible. When this occurs, chordal-sparing mitral valve replacement is performed.
Results
The operative mortality for isolated mitral valve repair in degenerative disease is less than 1%; in contrast, most series report somewhat higher mortality for mitral valve replacement (1,62,63,76,77,78,79). In patients with degenerative mitral valve disease, preserved LV function, and minimal symptoms, long-term survival after repair is similar to that of age-matched patients in the general population (59). Long-term survival is influenced by patient age and ventricular dysfunction (34). Patients receiving mitral valve repair have better survival rates when compared to patients undergoing mitral valve replacement (62,63).
Durability of mitral valve repair for degenerative disease is excellent. In patients with posterior leaflet prolapse, 10- and 20-year freedoms from reoperation exceed 90% (34,59,60,64). In contrast, 10- and 20-year freedoms from reoperation after correction of anterior prolapse are 80% to 90% (34,59,60,64). Increased surgical experience and advances in surgical technique are responsible for recent improvements in repair durability (60). However, these reoperation rates likely overestimate repair durability; some patients develop recurrent MR but do not undergo cardiac reoperation (80). Therefore, annual echocardiographic surveillance is indicated in all patients after mitral valve repair for degenerative disease.
Ischemic and Cardiomyopathic Mitral Regurgitation
Etiology and Pathology
Ischemic and cardiomyopathic MR occurs in patients with normal mitral leaflets and chordae and no significant intrinsic mitral valve disease. Rather, changes in ventricular, annular, and/or papillary muscle geometry cause leaflet malcoaptation and resultant MR (4,80,81,82,83). Ischemic MR occurs as a consequence of coronary artery disease and should be distinguished from coexisting organic mitral valve disease and coronary artery disease (4,84,85). Ischemic MR may be caused by papillary muscle rupture or elongation, which cause leaflet flail or prolapse. More commonly, however, patients have restricted leaflet motion caused by changes in ventricular and annular geometry; this is termed functional MR. Patients with functional ischemic MR generally have completed MIs in the distribution of the right or circumflex coronary arteries (4,81,82,83,84,85). Regional changes in ventricular geometry and function, including apical and posterior displacement of the papillary muscles, cause leaflet restriction and tenting, which usually affects the
posterior leaflet most severely and causes a central or posterior jet of MR (4,81



posterior leaflet most severely and causes a central or posterior jet of MR (4,81
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree