Introduction
In mitral valve disease, symptomatic status, ventricular functional status, and the kind of operation which will ultimately be performed all affect the indication for valve surgery. This chapter will integrate these aspects into a strategy for surgical correction. It should be noted that in surgery for valve disease there are few large controlled trials of therapy. Indeed, in the most recent (2006) AHA/ACC Guidelines for the Management of Patients with Valvular Heart Disease no recommendation is based on a level of evidence “A.”1 Most knowledge of the response of valve disease to surgery accrues from reports of surgical outcome in both selected and unselected patients.
Surgical objectives
Like all valvular lesions, mitral regurgitation imposes a hemodynamic overload on the heart. Ultimately, this overload can only be corrected by surgically restoring valve competence. For valve surgery in general, the timing of surgery has two opposing tenets. First, since surgery has an operative risk and, if a prosthesis is inserted, imposes the risks inherent to valve prosthesis, surgery should be delayed for as long as possible. Second, surgery which is delayed until the hemodynamic overload has caused irreversible left ventricular dysfunction will result in a suboptimal outcome. In some patients, far advanced left ventricular dysfunction may militate against operating at all.
The timing of valve surgery is made even more complex in mitral regurgitation since frequently valve repair rather than valve replacement can be effected. Because valve repair does not involve the use of a valvular prosthesis and because it also helps to preserve left ventricular function, it is applicable at the two ends of the spectrum of mitral regurgitation. Repair might be considered in asymptomatic patients with normal left ventricular function because the disease could be cured then without the need for intense follow-up and without the use of a valve prosthesis.2 At the other end of the spectrum, patients with severe impairment of left ventricular function who might not be candidates for mitral valve replacement with chordal disruption might have a good result from mitral valve repair.3 However, for most patients, mitral valve surgery is performed for the relief of symptoms or for prevention of worsening of asymptomatic left ventricular dysfunction.
Etiology
The mitral valve apparatus consists of the mitral valve annulus, the valve leaflets, the chorda tendineae, and the papillary muscles. Abnormalities of any of these structures may cause mitral regurgitation. The common causes of mitral regurgitation include infective endocarditis, the mitral valve prolapse syndrome with myxomatous degeneration of the valve, spontaneous chordal rupture, rheumatic heart disease, collagen disease such as Marfan’ s syndrome and coronary artery disease leading to papillary muscle ischemia or necrosis. These etiologies of mitral regurgitation are important especially with regard to surgical correction. For instance, the spontaneous rupture of a posterior chorda tendinea leads to mitral valve repair in almost 100% of cases. On the other hand, severe rheumatic deformity of the valve which has led to mitral regurgitation may be irreparable, necessitating mitral valve replacement.
Pathophysiology
Hemodynamic phases of mitral regurgitation
Figure 54.1 depicts the pathophysiologic phases of mitral regurgitation.4 In the acute phase, such as might occur with spontaneous chordal rupture, there is sudden volume overload on both the left ventricle and left atrium. The regurgitant volume, together with the venous return from the pulmonary veins, distends both chambers. Distension of the left ventricle increases use of the Frank – Starling mechanism by which increased sarcomere stretch increases end-diastolic volume modestly and also increases left ventricular stroke work. The new orifice for left ventricular ejection (the regurgitant pathway) facilitates left ventricular emptying and end-systolic volume decreases. Acting in concert, these two effects increase ejection fraction and total stroke volume. However, as shown in Figure 54.1, panel A, if only 50% of the total stroke volume is ejected into the aorta there is a net loss of 30% of the initial forward stroke volume. At the same time, volume overload on the left atrium increases left atrial pressure. At this point, the patient suffers from low output and pulmonary congestion and appears to be in left ventricular failure although left ventricular muscle function is either normal or even augmented by sympathetic reflexes. Acute severe mitral regurgitation may lead to shock and pulmonary edema, requiring intra-aortic balloon counterpulsation and urgent mitral valve repair or replacement. However, if the patient can be maintained in a relatively stable condition, he or she may then enter the chronic compensated phase (Fig. 54.1, panel B) within 3–6 months.
Figure 54.1 (a) Normal hemodynamic state compared to acute mitral regurgitation (AMR). In AMR, total stroke volume and ejection performance increase as preload is increased and afterload is reduced. However, forward stroke volume is reduced and left atrial pressure is increased. (b) AMR compared to chronic compensated mitral regurgitation (CCMR). In CCMR, increased end-diastolic volume permitted by eccentric hypertrophy increases both total and forward stroke volume. Enlargement of atrium and ventricle allows increased volume to be accommodated at lower filling pressure. Increase in afterload toward normal in this state of compensation reduces ejection performance slightly. (c) Chronic decompensated mitral regurgitation (CDMR) compared with CCMR contractile function is reduced and afterload is increased in CDMR. Both reduce ejection performance and forward cardiac output. There is further cardiac dilation in CDMR, leading to worsening mitral regurgitation, further compromising pump function by reducing forward stroke volume and increasing filling pressure. CF, contractile function; EDV, end-diastolic volume; E F, ejection fraction; ESS, end-systolic stress; ESV, end-systolic volume; FSV, forward stroke volume; LA, left atrial pressure; N, normal hemodynamic state; RF, regurgitant fraction; SL, sarcomere length. (Reproduced with permission from Carabello.4)
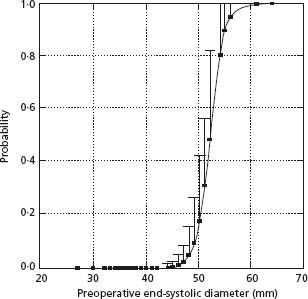
In the chronic compensated phase of mitral regurgitation, eccentric cardiac hypertrophy, in which sarcomeres are laid down in series, allows enlargement of the left ventricle, enhancing its total volume pumping capacity. Total stroke volume is increased, allowing normalization of forward stroke volume. Enlargement of the left atrium accommodates the volume overload at a lower pressure, eliminating pulmonary congestion. In this phase the patient may be remarkably asymptomatic, able to perform normal daily activities, and can even engage in sporting events of modest physical demands.5
The patient may remain in the compensated phase for months or years. However, eventually the persistent volume overload leads to a decline in left ventricular function (Fig. 54.1, panel C). A loss of myofibrils or an insensitivity to cyclic AMP may be responsible, at least in part, for loss of left ventricular contractility.6,7 In this phase, left ventricular end-systolic volume increases because reduced force of contraction results in poor left ventricular emptying, forward stroke volume falls, and left ventricular dilation may worsen the mitral regurgitation. At this time there is re-elevation of the left atrial pressure, resulting again in pulmonary congestion. Of note, the still favorable loading conditions of mitral regurgitation (increased preload and normal afterload) permit a “normal” ejection fraction even though left ventricular dysfunction has developed.
Importance of the mitral valve apparatus
Although the contribution of the mitral valve apparatus to left ventricular function was noted by Rushmer and Lillehei decades ago,8,9 its physiologic significance and impact on patient care have only recently received widespread appreciation. It is quite clear that the mitral valve apparatus has a wider role than to simply prevent mitral regurgitation. Rather, the apparatus is an integral part of the left ventricular internal skeleton. In early systole, tugging on the apparatus by the chorda tendineae may shorten the major axis while lengthening the minor axis, in turn augmenting preload there during ejection systole. In addition, the apparatus helps to maintain the normal and efficient ellipsoid shape of the left ventricle.
Transsection of the chordae causes an immediate fall in left ventricular function.10 Until the importance of chordal preservation during mitral valve surgery was recognized, ejection fraction almost always fell following surgery. This fall was attributed to increased afterload from surgical closure of the low-impedance pathway which preopera-tively had facilitated ejection into the left atrium. However, it is now clear that closure of the same low-impedance pathway in which chordal integrity is maintained results in no fall in ejection fraction or only a modest decline, suggesting that the increased postoperative load theory is not the sole mechanism for why ejection fraction falls.3 In fact, chordal preservation can actually effect a lowering of systolic wall stress (afterload) instead of an increase as left ventricular radius decreases following surgery (stress = pressure × radius/2× thickness).11 Thus, chordal integrity should be maintained whenever possible12–14; in fact, a randomized study demonstrated that maintenance of just the posterior apparatus lowers mortality and leads to superior postoperative function compared to posterior and anterior chordal transection.15
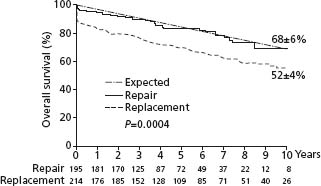
Apart from the benefits on left ventricular function, if mitral valve repair instead of a replacement can be performed, operative mortality is lower (Fig. 54.2), postoperative survival is better and the need for anticoagulation is removed while thromboembolism remains low.13,16–18 Long-term outcome is excellent and repairs are durable. In one study only seven of 162 patients required reoperation a mean of 17 years after repair.19 Even if the mitral valve is so badly damaged that a mitral valve prosthesis must be inserted, chordal preservation, especially of the posterior chords, can usually be performed resulting in better ventricular function than if all the chords were removed.12 Unfortunately, despite recognition of the importance of the mitral valve apparatus, mitral repair is only performed in about 50% of all operations for mitral regurgitation, varying from 0% in some institutions to 90% in others.
Figure 54.2 Postoperative survival following mitral valve repair is compared to that of mitral valve replacement.
Indications for surgery
Severity of mitral regurgitation
Under most circumstances only severe mitral regurgitation is corrected surgically. Mild to moderate regurgitation (regurgitant fraction <40%) neither causes symptoms nor leads to left ventricular dysfunction even over a protracted period of time in the absence of other cardiac disease. Severity is difficult to ascertain by physical examination alone, especially in acute mitral regurgitation. As noted above, in acute mitral regurgitation there has been no time for cardiac dilation to occur. Thus, palpation of the precordium does not reveal a hyperdynamic left ventricular impulse. Although the murmur of mitral regurgitation is present, severity cannot be gauged from its intensity. In most cases of severe mitral regurgitation an S3 should be present. This finding does not necessarily indicate heart failure but may simply be the result of a large regurgitant volume filling the left ventricle under a higher than usual left atrial pressure. In chronic mitral regurgitation there should be evidence on physical examination of an enlarged hyperdynamic left ventricle unless the patient’ s size or habitus makes physical examination difficult. Failure to find evidence of an enlarged heart suggests that the mitral regurgitation is neither severe enough or chronic enough to cause left ventricular enlargement.
In chronic severe mitral regurgitation, the chest radiograph should also show cardiac enlargement and the electrocardiogram is likely to demonstrate left atrial abnormality and left ventricular hypertrophy.
In most cases, quantitation of regurgitant severity is estimated during echocardiography with Doppler interrogation of the mitral valve. In acute mitral regurgitation, transthoracic echocardiography may underestimate regurgitant severity.20 In such cases, transesophageal echocar-diography is helpful. It should be noted that Doppler flow studies visually demonstrate blood flow velocity across the mitral valve and not true flow. Because of this, both under-and overestimation of regurgitant severity is possible. Flow mapping, which expresses the regurgitant jet in terms relative to left atrial size, has been used extensively. However, limitations of this method are well known and the technique is semiquantitative at best.20,21 Other methods, such as the proximal isovelocity surface area, have been employed experimentally and in clinical investigation.23–25 In using proximal isovelocity surface area to estimate regurgitation flow, the area of convergence of the regurgitant jet on the ventricular side of the mitral valve is measured at the point of aliasing. By multiplying proximal isovelocity surface area by the known aliasing velocity, actual flow is obtained, which should be a better indication of regurgitant severity and even outcome. Indeed, in one prospective study a regurgitant orifice area of >0.4cm2 predicted a poorer prognosis than when orifice area was smaller.26 However, the convergence pattern is often diffi-cult to pinpoint clinically and is not applicable in many cases. As with mitral valve repair, practice varies from center to center with some centers routinely accurately quantifying the severity of disease27 while others rely upon a visual estimation.
When regurgitation severity is in doubt because of discordance between left ventricular size and the regurgitant signal, i.e. a small left ventricle and left atrium suggesting mild disease and a Doppler signal suggesting severe disease, the issue often can be resolved at cardiac catheterization. During cardiac catheterization, hemodynamics and a left ventriculogram give additional although also imperfect information about the degree of mitral regurgitation. The left ventriculogram, unlike the Doppler study, visualizes actual flow of contrast media from the left ventricle into the left atrium. Care must be taken to inject enough contrast agent (at least 60 mL) to opacify both the enlarged left ventricle and left atrium of mitral regurgitation. Coronary arteriography is also performed at cardiac catheterization if there is any suspicion of an ischemic etiology for mitral regurgitation or when risk factors for coronary disease co-exist.
Acute mitral regurgitation
In almost all cases of severe acute mitral regurgitation, the patient is symptomatic. The acute hemodynamic changes noted above cause decreased forward output and sudden left atrial hypertension, resulting in pulmonary congestion, reduced forward flow, and the symptoms of dyspnea, orthopnea, exercise intolerance, and fatigue. Vasodilator therapy may be successful in alleviating symptoms by preferentially increasing forward flow while simultaneously decreasing left ventricular size, partially restoring mitral valve competence.28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


