Mitral Stenosis
D. Elizabeth Le, MD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Exertional dyspnea and fatigue.
Exertional dyspnea and fatigue.
![]() Opening snap, diastolic rumble murmur, loud S1, presystolic accentuated murmur.
Opening snap, diastolic rumble murmur, loud S1, presystolic accentuated murmur.
![]() Right ventricular heave and loud P2 if pulmonary hypertension and right heart failure are present.
Right ventricular heave and loud P2 if pulmonary hypertension and right heart failure are present.
![]() A2-OS interval ≤ 80 ms in severe mitral stenosis.
A2-OS interval ≤ 80 ms in severe mitral stenosis.
![]() Sinus rhythm or atrial fibrillation, notched P wave or P mitrale in leads II and III and/or biphasic P wave in V1, right axis deviation, high amplitude of P wave in lead II, and large R wave in V1 on electrocardiography.
Sinus rhythm or atrial fibrillation, notched P wave or P mitrale in leads II and III and/or biphasic P wave in V1, right axis deviation, high amplitude of P wave in lead II, and large R wave in V1 on electrocardiography.
![]() Flattening of left atrial border and/or double density, elevated left main bronchus, enlarged pulmonary arteries, and Kerley B lines on chest radiography.
Flattening of left atrial border and/or double density, elevated left main bronchus, enlarged pulmonary arteries, and Kerley B lines on chest radiography.
![]() Thickened and/or calcified mitral leaflets and subvalvular apparatus resulting in “hockey-stick” motion of the anterior leaflet and fusion of commissures resulting in fish-mouth appearance of the mitral valve on two- and three-dimensional echocardiography.
Thickened and/or calcified mitral leaflets and subvalvular apparatus resulting in “hockey-stick” motion of the anterior leaflet and fusion of commissures resulting in fish-mouth appearance of the mitral valve on two- and three-dimensional echocardiography.
![]() Increased transmitral valve gradient and reduced mitral valve area by planimetry, pressure half-time, continuity equation, or proximal isovelocity surface area method of quantification on Doppler echocardiography.
Increased transmitral valve gradient and reduced mitral valve area by planimetry, pressure half-time, continuity equation, or proximal isovelocity surface area method of quantification on Doppler echocardiography.
 General Considerations
General Considerations
Mitral stenosis is a condition where the mitral valve area is reduced, causing obstruction of blood flow from the left atrium into the left ventricle during left ventricular diastole, which can lead to elevated left atrial pressure resulting in pulmonary hypertension, pulmonary edema, and right heart failure. The condition becomes clinically evident when the mitral valve area is reduced to approximately 2 cm2. Mitral stenosis occurs predominantly in adults and is one of the sequelae of rheumatic fever in about 90% of cases. Only about 50–70% patients recall having had antecedent group A β-hemolytic streptococcal tonsillopharyngitis. It has an indolent course, and the latent period can be as long as 40 years after the initial episode of rheumatic fever.
Rheumatic fever is a major public health problem in developing countries. The prevalence in developing countries is 2.2 to 2.3 per 1000 using clinical screening compared to 0.5 per 1000 in developed countries. If echocardiography is used to screen, the prevalence increases to 21.5 to 30.4 per 1000 in underdeveloped countries. Despite this fourfold difference, the prevalence in Western countries has not decreased substantially because of the increased rate of immigration from developing countries. Mitral stenosis still accounts for 10% of native valve pathology. The pattern of valvular involvement is associated with the rate of recurrent or reactivation of streptococcal infection and thus varies geographically. Individuals in developing countries are more likely to suffer from multiple episodes of rheumatic fever and thus become symptomatic at an earlier age compared to individuals in industrialized countries, who often do not present until they are ≥ 45 years old. Patients from developing countries tend to have more severe stenosis but less leaflet calcification and less atrial fibrillation. Due to the shorter latent period, younger patients tend to have more commissural thickening and pliable valves, whereas older patients with a longer latent period tend to have more calcified valves. A subgroup of patients, pregnant women, often present during the second trimester of pregnancy because increases in intravascular blood volume, cardiac output, and heart rate can make the impediment to flow more pronounced.
A. Anatomy
The mitral valve has two leaflets, anterior and posterior, which come together at the anterior and posterior commissures. The anterior leaflet occupies one-third of the saddle-shaped annular circumference, and the posterior occupies two-thirds of the circumference. Primary, secondary, and tertiary chordae tendineae originate from both the anterolateral and posteromedial papillary muscles of the left ventricle and insert into margin and base of both the anterior and posterior leaflets. A competent mitral valve requires precise and concerted motion of the leaflets, chordae, and left ventricular and atrial contraction. Alteration of any components of this complex geometry can result in stenosis, insufficiency, or both (Figure 19–1).
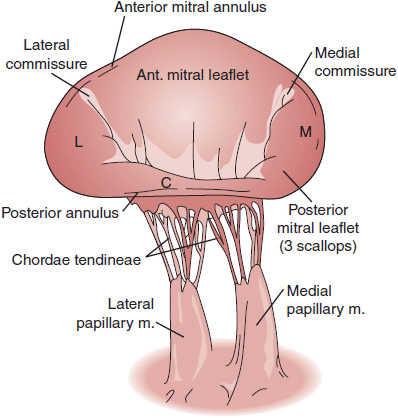
![]() Figure 19–1. The mitral valve apparatus. The anterior mitral leaflet attaches to a smaller portion of the circumference of the annulus than the posterior mitral leaflet, but the anterior leaflet is longer. The posterior leaflet consists of three segments designated the lateral (L or P1), central (C or P2), and medial (M or P3) scallops. Both leaflets attach to both the medial and lateral papillary muscles. (From Otto CM. Textbook of Clinical Echocardiography, 4th ed. Philadelphia: Elsevier; 2009, with permission.)
Figure 19–1. The mitral valve apparatus. The anterior mitral leaflet attaches to a smaller portion of the circumference of the annulus than the posterior mitral leaflet, but the anterior leaflet is longer. The posterior leaflet consists of three segments designated the lateral (L or P1), central (C or P2), and medial (M or P3) scallops. Both leaflets attach to both the medial and lateral papillary muscles. (From Otto CM. Textbook of Clinical Echocardiography, 4th ed. Philadelphia: Elsevier; 2009, with permission.)
B. Etiology
Mitral stenosis is predominantly due to organic valve pathology and less commonly due to functional valve pathology where the mitral leaflets are anatomically normal but their movement is altered. Approximately 90% of organic native valve mitral stenosis cases are associated with rheumatic fever or rheumatic heart disease, where the autoimmune response to group A streptococcal infection results in commissural thickening and fusion, valve and subvalvular apparatus thickening, calcification, and fusion, and consequently, restricted motion of the valve leaflets (Figure 19–2). Infective endocarditis and severe mitral annular calcification account for about 3% of cases. Other conditions that have been associated with valve fibrosis are systemic lupus erythematous, rheumatoid arthritis, malignant carcinoid, and exposure to medications with direct serotonin receptors activation (ergotamine and methysergide for migraine headaches, fenfluramine and chlorphentermine for weight reduction, and pergolide and cabergoline for Parkinson disease). In < 1% of cases, mitral stenosis is due to congenital abnormalities and thus will present during infancy or childhood. These rare entities include supravalvar mitral ring, which is an abnormal connective tissue band located above the annulus or is adherent to the annulus and valve, hypoplasia of the mitral annulus, fusion of the commissures, a double orifice mitral valve, and a parachute mitral valve, where chordae from both the anterior and posterior leaflets insert into one papillary muscle. Another rare anomaly, cor triatriatum, can result in stenotic physiology without valve involvement. In this condition, a membrane or a fibromuscular band separates the left atrium into two compartments. This abnormal tissue may vary in size and shape, may have fenestrations, or can be funnel-shaped, which restricts blood flow from the left atrium into the left ventricle.
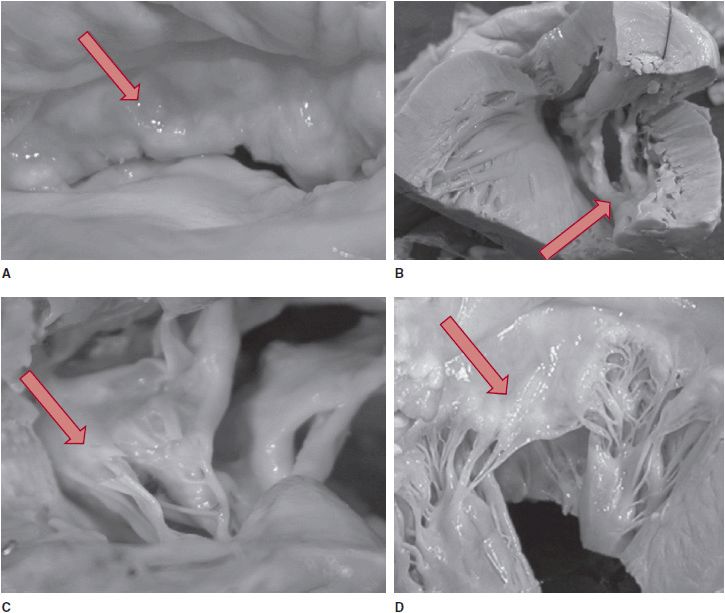
![]() Figure 19–2. Postmortem specimens of a rheumatic mitral valve and normal mitral valve. A: Thickened and nodular leaflet in rheumatic mitral valve. B: Calcified and fused commissure, fish-mouth shaped leaflets. C: Thickened, fused, and shortened subvalvular apparatus. D: Normal mitral leaflet and chordae tendineae. (Adapted, with permission, from Chandrashekhar Y, et al. Lancet. 2009;374:1271.)
Figure 19–2. Postmortem specimens of a rheumatic mitral valve and normal mitral valve. A: Thickened and nodular leaflet in rheumatic mitral valve. B: Calcified and fused commissure, fish-mouth shaped leaflets. C: Thickened, fused, and shortened subvalvular apparatus. D: Normal mitral leaflet and chordae tendineae. (Adapted, with permission, from Chandrashekhar Y, et al. Lancet. 2009;374:1271.)
Functional native mitral valve stenosis is rare and can result from a mass, such as a left atrial myxoma or a large left atrial thrombus, advancing across the mitral annulus and occupying a portion of the mitral orifice during left ventricular diastole. Acquired mitral stenosis can also be iatrogenic where a portion of the mitral leaflet is inadvertently sutured to annular tissue during aortic or mitral valve surgery. Impingement of the anterior leaflet of the mitral valve with the CoreValve during percutaneous intervention to treat severe aortic stenosis resulting in stenosis has been reported.
Mitral stenosis can also involve prosthetic valves, where a bioprosthetic mitral valve can calcify over time with or without pannus overgrowth and cause similar pathophysiology seen in native valves. Mechanical mitral valves can also exhibit stenotic physiology when leaflet or poppet motion is impeded by thrombus, vegetation, or pannus.
C. Pathophysiology
The normal mitral valve area is 4–6 cm2. Mild mitral stenosis occurs when the mitral valve area is reduced to 2 cm2 and becomes severe when the area is ≤ 1 cm2. In normal conditions, during early left ventricular diastole, blood flow from the left atrium into the left ventricle occurs from passive filling, is rapid, and peaks early, followed by diastasis, until active filling occurs from atrial contraction. Equalization of left atrial and left ventricular pressure occurs during middiastole. In the presence of mitral stenosis, the rate of passive filling is reduced and sustained throughout diastole, such that at end-diastole, left atrial pressure is higher than left ventricular pressure (Figure 19–3).
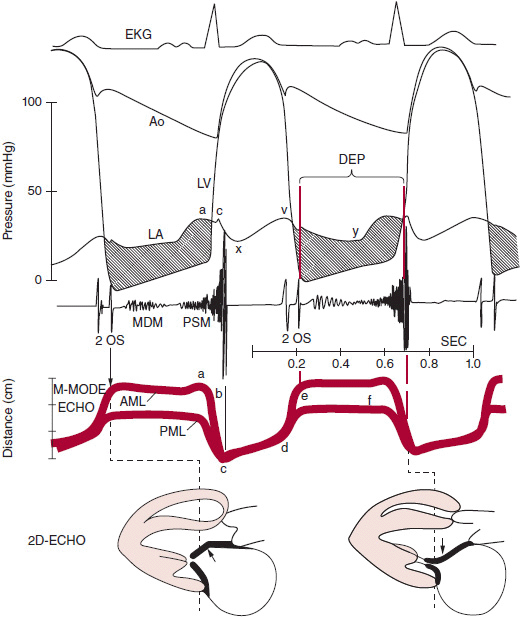
![]() Figure 19–3. Correlation of electrocardiogram (EKG), hemodynamic tracings, cardiac auscultation, M-mode, and two-dimensional (2D) echocardiography (ECHO) of mitral stenosis. The left atrium (LA) pressure tracing shows a prominent a wave and v wave. The atrioventricular pressure gradient (shaded area) is sustained throughout diastole with elevated LA pressure. The E-F slope is flat on M-mode recording of the mitral leaflets. AML, anterior mitral leaflet; AO, aorta; DFP, diastolic filling period; LV, left ventricle; MDM, mid-diastolic murmur; OS, opening snap; PML, posterior mitral leaflet; PSM, presystolic murmur. (Adapted, with permission, from Heger JW, et al. Cardiology, 3rd ed. Baltimore: Williams & Wilkins; 1994.)
Figure 19–3. Correlation of electrocardiogram (EKG), hemodynamic tracings, cardiac auscultation, M-mode, and two-dimensional (2D) echocardiography (ECHO) of mitral stenosis. The left atrium (LA) pressure tracing shows a prominent a wave and v wave. The atrioventricular pressure gradient (shaded area) is sustained throughout diastole with elevated LA pressure. The E-F slope is flat on M-mode recording of the mitral leaflets. AML, anterior mitral leaflet; AO, aorta; DFP, diastolic filling period; LV, left ventricle; MDM, mid-diastolic murmur; OS, opening snap; PML, posterior mitral leaflet; PSM, presystolic murmur. (Adapted, with permission, from Heger JW, et al. Cardiology, 3rd ed. Baltimore: Williams & Wilkins; 1994.)
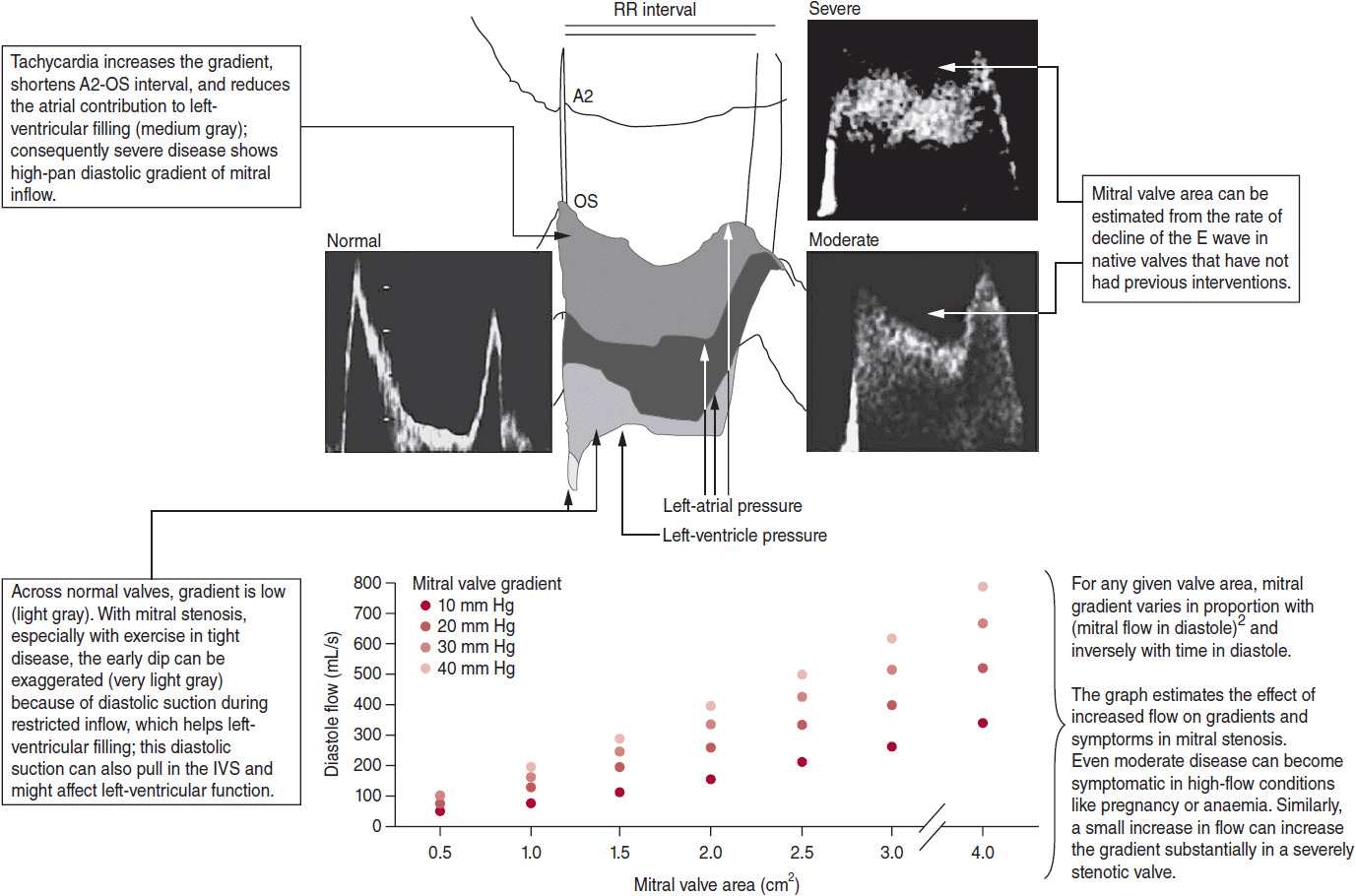
Within a cardiac cycle, the diastolic filling period (seconds per cycle) is the time between mitral valve opening and closure. The diastolic filling time is determined using the following formula:
Mitral flow is a function of both the cardiac output and diastolic filling time and is calculated using the following equation:
![]()
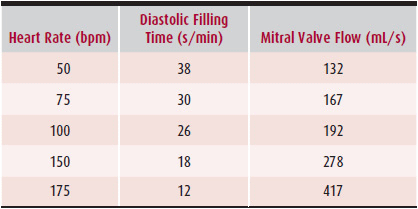
During each minute, the diastolic filling period averages about 30–32 seconds. As heart rate increases, more time of the 1 minute is devoted to systolic ejection and isovolumetric contraction and relaxation, thus leading to a reduction in the diastolic filling period (Table 19–1). At rest, the diastolic flow across the mitral valve is about 200 mL/s. During exercise, cardiac output can triple, and along with tachycardia and the compensatory reduction in diastolic filling, transmitral flow and gradient will increase. Assuming no change in left atrial and left ventricular compliance, as the mitral valve area decreases, left atrial pressure and transmitral gradient will increase disproportionately with mitral flow (Figure 19–4). Chronically elevated left atrial pressure results in left atrial enlargement, which can lead to paroxysmal or permanent atrial fibrillation. Rapid atrial fibrillation reduces the diastolic filling time and cardiac output, which in turn, perpetuates the vicious circle by further increasing left atrial pressure. Chronic elevation of left atrial pressure causes a rise in pulmonary venous pressure and eventually pulmonary arterial pressure. When left atrial pressure exceeds 25 mm Hg, pulmonary edema develops. Consequently, chronic pulmonary arterial hypertension leads to right ventricular pressure overload and then volume overload when the right heart fails. Over time, the pulmonary vasculature becomes permanently damaged, leading to fixed pulmonary hypertension and elevation of pulmonary vascular resistance.
Table 19–1. Relation Between Heart Rate, Diastolic Filling Time, and Mitral Valve Flow with a Constant Cardiac Output of 5 L/min
![]() Figure 19–4. Pathophysiology of mitral stenosis. (Adapted, with permission, from Chandrashekhar Y, et al. Lancet. 2009;374:1271.)
Figure 19–4. Pathophysiology of mitral stenosis. (Adapted, with permission, from Chandrashekhar Y, et al. Lancet. 2009;374:1271.)
Mitral stenosis is associated with increased levels of prothrombotic metabolites. This hypercoagulable milieu poses a high risk of left atrial and left atrial appendage thrombus formation. Other factors that independently predict thrombosis in mitral stenosis are ≥ 1 year duration of atrial fibrillation, left atrial size ≥ 4.5 cm, left atrial spontaneous echo contrast on echocardiography, and a prior thromboembolic event.
Balghith M, et al. Transcatheter aortic valve implantation (core valve) prosthesis complicated by mitral stenosis. J Saudi Heart Assoc. 2012;24(2):149–50.
Iung B. Mitral stenosis still a concern in heart valve diseases. Arch Cardiovasc Dis. 2008;101:597–9. [PMID: 19056064]
Roeder HA, et al. Maternal valvular heart disease in pregnancy. Obstet Gynecol Surv. 2011;66(9):561–71. [PMID: 22088233]
 Clinical Findings
Clinical Findings
A. Symptoms & Signs
1. Dyspnea and fatigue—The classic symptoms of mitral stenosis are dyspnea and fatigue during exercise or in the setting of any condition that results in sinus tachycardia such as hyperthyroidism, hypovolemia, anemia, fever/infection, emotional stress, pregnancy, or exposure to sympathomimetic agents. Those with more advanced disease can experience shortness of breath at rest. In addition, patients are predisposed to developing atrial fibrillation, and the loss of atrial contraction and synchronized contraction with the left ventricle during each cardiac cycle can exacerbate dyspnea on exertion or even produce shortness of breath at rest, especially in patients with left ventricular dysfunction. Chest pain is rare, but patients may have intermittent or sustained palpitations from paroxysmal or permanent atrial fibrillation. One of the most devastating morbidities associated with mitral stenosis is embolic cerebral vascular accident, which occasionally can be the presenting symptom.
2. Congestive heart failure—As mitral stenosis becomes more severe, elevated left atrial pressure causes pulmonary vascular congestion. Pulmonary edema usually is manifested by dyspnea. In the early stages, pulmonary edema may occur only during exertion, and as the disease progresses, pulmonary edema occurs at rest. Occasionally, acute pulmonary edema can occur with exercise. Other signs of left heart failure include weight gain, orthopnea, and paroxysmal nocturnal dyspnea. In the late stages of mitral stenosis, there is irreversible adverse remodeling of the pulmonary vasculature, which results in elevated pulmonary artery pressure and pulmonary vascular resistance. Subsequently, right heart failure ensues when right heart hypertrophy can no longer adapt to the high pulmonary and right ventricular pressures. Symptoms of right heart failure include exercise intolerance, dyspnea, abdominal fullness, and lower extremity edema.
3. Hemoptysis—When severe mitral stenosis leads to severe pulmonary hypertension, rarely the bronchial veins can rupture and cause hemoptysis.
B. Physical Examination
1. Arterial pulses—In severe stenosis, when stroke volume is reduced, pulses are diminished.
2. Rhythm—In the early stages of mitral stenosis, patients have normal sinus rhythm. In moderate to severe mitral stenosis, elevated left atrial pressure and atrial tissue fibrosis can lead to atrial arrhythmia, predominantly in the form of atrial fibrillation, where heart sounds will be rapid and irregularly irregular.
3. Opening snap—The opening snap (OS) is due to the abrupt opening of the mitral leaflets during left ventricular diastole, where the leaflets are still relatively mobile. It may not be detected if the valve is severely calcified and restricted. The OS is heard best at the base of the heart but can also be audible at the apex. The duration between aortic valve closure and OS, the A2-OS interval, can be used to determine stenosis severity. The astute clinician can estimate the duration of this interval on auscultation. An A2-OS duration of < 80 ms is indicative of severe mitral stenosis with a corresponding left atrial pressure of at least 25 mm Hg. As left atrial pressure rises, the OS occurs earlier, leading to a shorter A2-OS interval (see Figure 19–3).
4. Diastolic rumble murmur—The diastolic murmur in mitral stenosis is due to turbulent flow across the mitral valve. This murmur is low-pitch and is heard best at the apex using the bell of the stethoscope when the patient lies in the left lateral decubitus position. The diastolic murmur can also be more prominent after exercise, although tachycardia results in a shorter diastolic filling time, which may make auscultation more challenging. Maneuvers that increase venous return accentuate the diastolic murmur and shorten the A2-OS interval, whereas maneuvers that decrease venous return decrease the murmur and lengthen the A2-OS interval. The duration of the murmur, and not the amplitude, correlates with stenosis severity. In mild stenosis, the diastolic murmur is audible during late ventricular diastole when atrial systole occurs and represents “presystolic accentuation.” As the valve becomes more stenotic, the diastolic murmur is present throughout diastole. In very severe stenosis, the amplitude may be diminished significantly due to extremely low flow across the valve and may be inaudible.
5. Loud S1—Closure of minimally calcified and pliable mitral leaflets transmits as a loud S1. However, as calcification increases, the valve is less mobile and the S1 is soft.
6. Findings with pulmonary hypertension—A loud pulmonic valve closure (P2) can be audible in patients who have developed passive pulmonary hypertension. There may also be a systolic murmur from tricuspid regurgitation. If right ventricular hypertrophy is also present, a right ventricular heave may be palpable. Right heart systolic dysfunction can present as hepatomegaly, peripheral edema, and ascites.
7. Other findings—If there is mixed valve disease, the holosystolic murmur of chronic mitral regurgitation is present. If there is concomitant aortic regurgitation or pulmonary insufficiency, a high-pitch blowing diastolic murmur can be appreciated. If a patient has left ventricular systolic failure, elevated jugular venous pressure, pulmonary rales, and lower extremity edema are seen.
 Diagnostic Studies
Diagnostic Studies
A. Electrocardiography
The rhythm can be sinus or atrial fibrillation. A notched P wave or “P mitrale” in leads II and III and/or a biphasic P wave in lead V1 can be present, which reflect left atrial enlargement. If there is right ventricular enlargement and right ventricular failure, they manifest as right axis deviation, incomplete right bundle branch block, and tall R wave in V2 or deep S wave in V6. Right atrial enlargement is represented by high amplitude (≥ 2.5 mm) of the P wave in lead II (Figure 19–5).

![]() Figure 19–5. Electrocardiogram demonstrating right axis deviation (+90° to +180°, negative amplitude in lead I, and positive amplitude in lead aVF), left atrial enlargement (notched P wave in lead II and biphasic P wave in leads V1 and V2), right atrial enlargement (P-wave amplitude of 4 mm in lead II), and right ventricular hypertrophy (right axis deviation > 90°, tall R in V2, and prominent S wave in V6) seen in mitral stenosis. (From American College of Cardiology ECG-SAPII, with permission. Copyright © 1997.)
Figure 19–5. Electrocardiogram demonstrating right axis deviation (+90° to +180°, negative amplitude in lead I, and positive amplitude in lead aVF), left atrial enlargement (notched P wave in lead II and biphasic P wave in leads V1 and V2), right atrial enlargement (P-wave amplitude of 4 mm in lead II), and right ventricular hypertrophy (right axis deviation > 90°, tall R in V2, and prominent S wave in V6) seen in mitral stenosis. (From American College of Cardiology ECG-SAPII, with permission. Copyright © 1997.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


